Question: Please, solve both by Polymath and by hand! Analyze the material balance of the reactor in which the ammonia synthesis reaction reaches the equilibrium state:
Please, solve both by Polymath and by hand!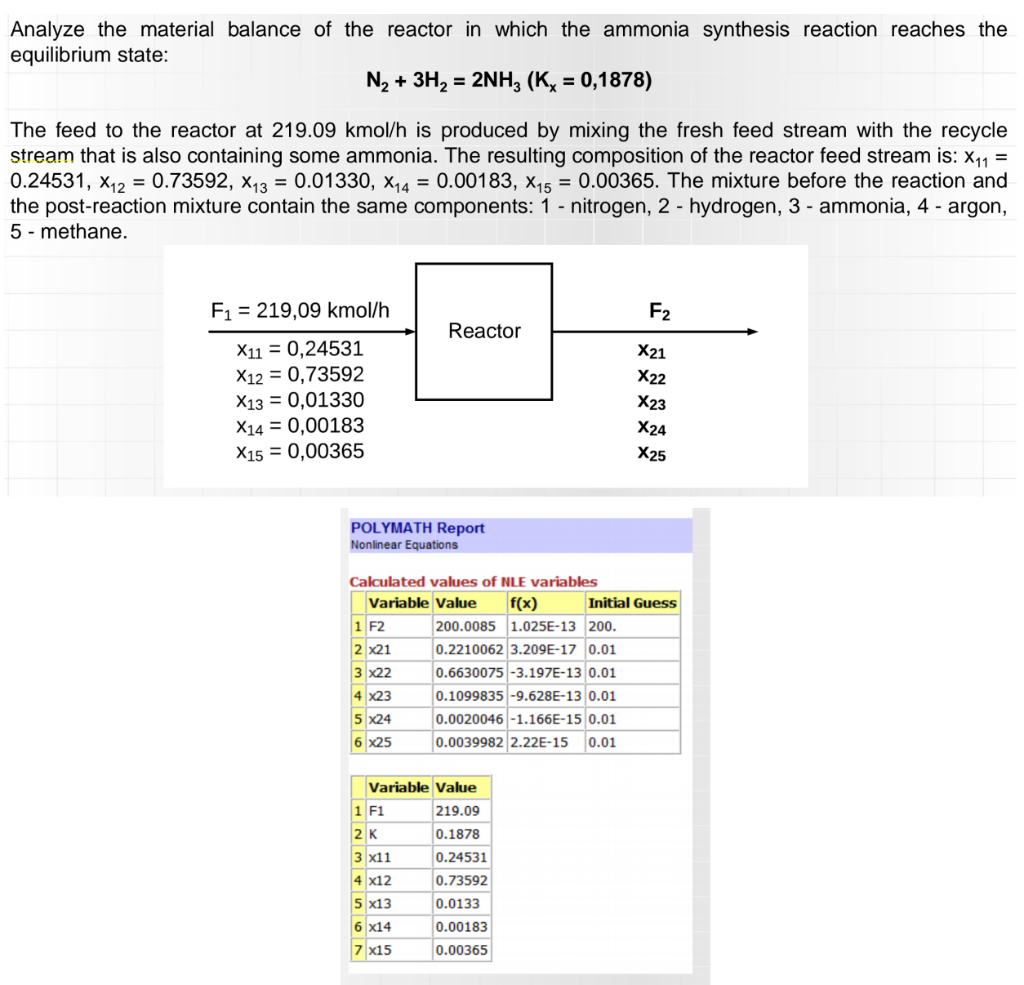
Analyze the material balance of the reactor in which the ammonia synthesis reaction reaches the equilibrium state: N2 + 3H2 = 2NH3 (Kx = 0,1878) The feed to the reactor at 219.09 kmol/h is produced by mixing the fresh feed stream with the recycle stream that is also containing some ammonia. The resulting composition of the reactor feed stream is: X11 = 0.24531, X12 = 0.73592, X13 = 0.01330, X14 = 0.00183, X15 = 0.00365. The mixture before the reaction and the post-reaction mixture contain the same components: 1 - nitrogen, 2 - hydrogen, 3 - ammonia, 4 - argon, 5 - methane. F1 = 219,09 kmol/h F2 Reactor X21 X22 X1 = 0,24531 X12 = 0,73592 X13 = 0,01330 X14 = 0,00183 X15 = 0,00365 X23 X24 X25 POLYMATH Report Nonlinear Equations Calculated values of NLE variables Variable Value f(x) Initial Guess 1 F2 200.0085 1.025E-13 200. 2 X21 0.2210062 3.209E-17 0.01 3 X22 0.6630075 -3.197E-13 0.01 4 X23 0.1099835 -9.628E-13 0.01 5 X24 0.0020046 -1.166E-15 0.01 6 x25 0.0039982 2.22E-15 0.01 0.1878 Variable Value 1 F1 219.09 |2K 3 x11 0.24531 4 x12 0.73592 5 x13 0.0133 6 X14 0.00183 7 x15 0.00365
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


