Question: please solve:) i appreciate the help! Consider these reactions, where M represents a generic metal. 1. 2M(s)+6HCl(aq)2MCl3(aq)+3H2(g)H1=928.0kJ 2. HCl(g)HCl(aq) H2=74.8kJ 3. H2(g)+Cl2(g)2HCl(g) H3=1845.0kJ 4. MCl3(s)MCl3(aq)
please solve:) i appreciate the help! 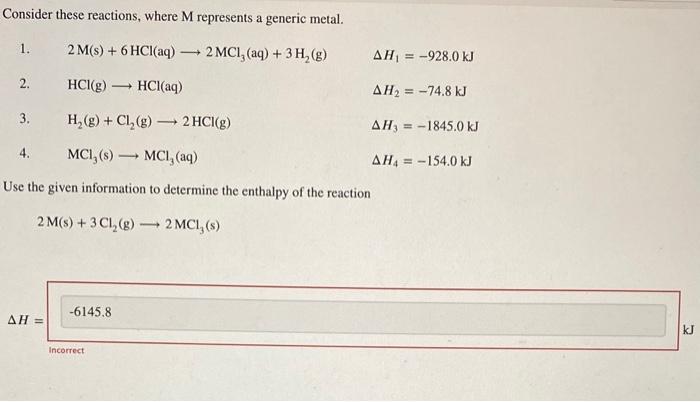

Consider these reactions, where M represents a generic metal. 1. 2M(s)+6HCl(aq)2MCl3(aq)+3H2(g)H1=928.0kJ 2. HCl(g)HCl(aq) H2=74.8kJ 3. H2(g)+Cl2(g)2HCl(g) H3=1845.0kJ 4. MCl3(s)MCl3(aq) H4=154.0kJ Use the given information to determine the enthalpy of the reaction 2M(s)+3Cl2(g)2MCl3(s)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


