Question: Please, solve it by hand or by Polymath. If your answer is wrong, I'll give you DISLIKE as a reward! The production of fertilizer grade
Please, solve it by hand or by Polymath. If your answer is wrong, I'll give you DISLIKE as a reward!
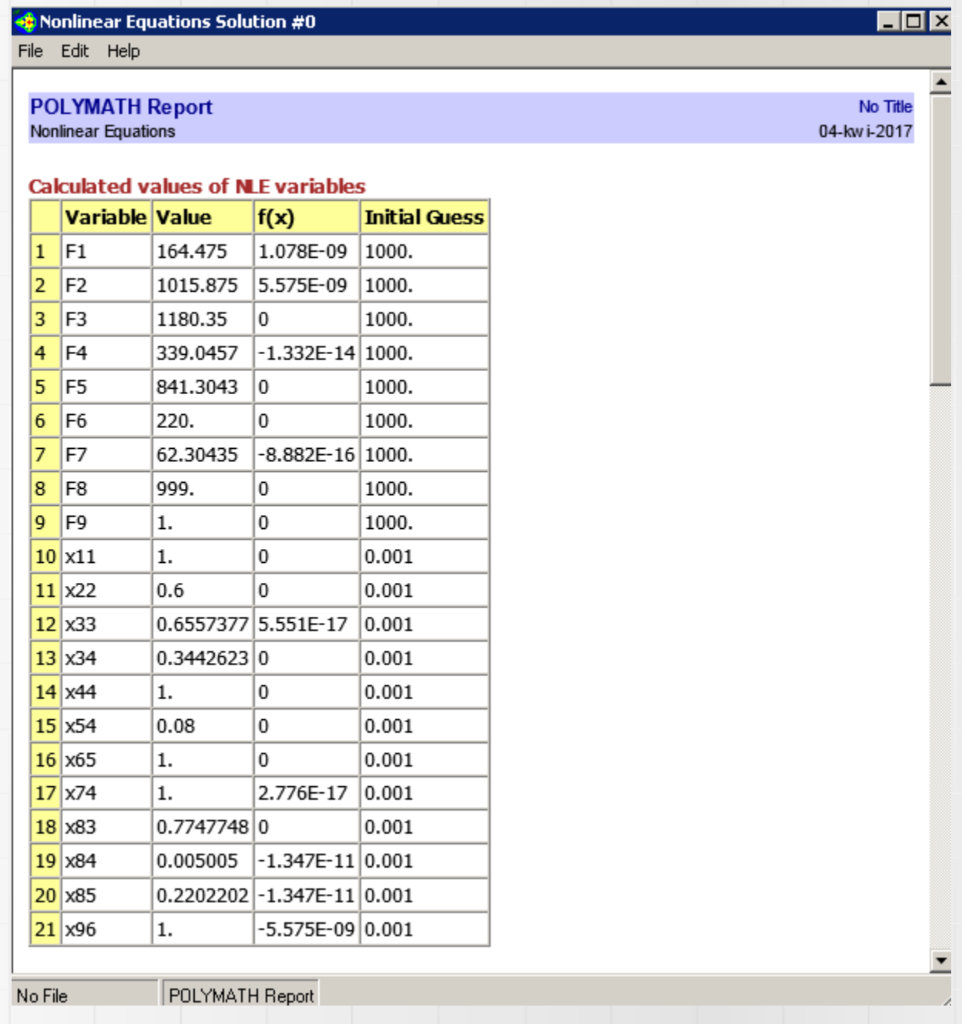
The production of fertilizer grade ammonium nitrate is carried out by neutralizing a 60 wt% solution of nitric acid with ammonia gas in a tubular reactor. The resulting ammonium nitrate stream is concentrated in a special tank to the concentration of 92 wt%. The concentrated melt is granulated together with the filler (CaCO3) in a drum granulator (where water is also re-evaporated), and the finished granules are conveyed to a fluidizer where they are sprayed with a conditioning agent. The finished product has the following composition: 77.4 wt% NH4NO3, 22.0 wt% CaCO3, 0.5 wt% H20 and 0.1 wt% conditioning agent. a MNH3=17 g/mol, MHNO3=63 g/mol, MNH4NO3=80 g/mol. Calculate the values and composition of all (mass) streams, if 1000 kg/h of finished fertilizer is obtained. Nonlinear Equations Solution #0 File Edit Help POLYMATH Report Nonlinear Equations No Title 04-kw i-2017 Calculated values of NE variables Variable Value f(x) Initial Guess 1 F1 164.475 1.078E-09 1000. 2 F2 1015.875 5.575E-09 1000. 3 F3 1180.35 0 1000. 4 F4 339.0457 -1.332E-14 1000. 5 F5 841.3043 0 1000. 6 F6 220. lo 1000. 7 F7 62.30435 -8.882E-16 1000. 8 F8 999. 1000. oo 9 F9 1. 1000. 10 x11 1. 0 0.001 11 X22 0.6 0 0.001 12 x33 13 X34 0.6557377 5.551E-17 0.001 0.3442623 0 0.001 1. 0 0.001 0.08 0 0.001 14 x44 15 x54 16 x65 1. 0 0.001 2.776E-17 0.001 17 x74 1. 18 X83 19 X84 0.7747748 0 0.001 0.005005 -1.347E-11 0.001 0.2202202 -1.347E-11 0.001 1. -5.575E-09 0.001 20 x85 21 X96 No File POLYMATH Report
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


