Question: please solve it by using (excel) only I don't want a manual solution In a sulfuric acid plant (figure 2), sulfur is burned in the
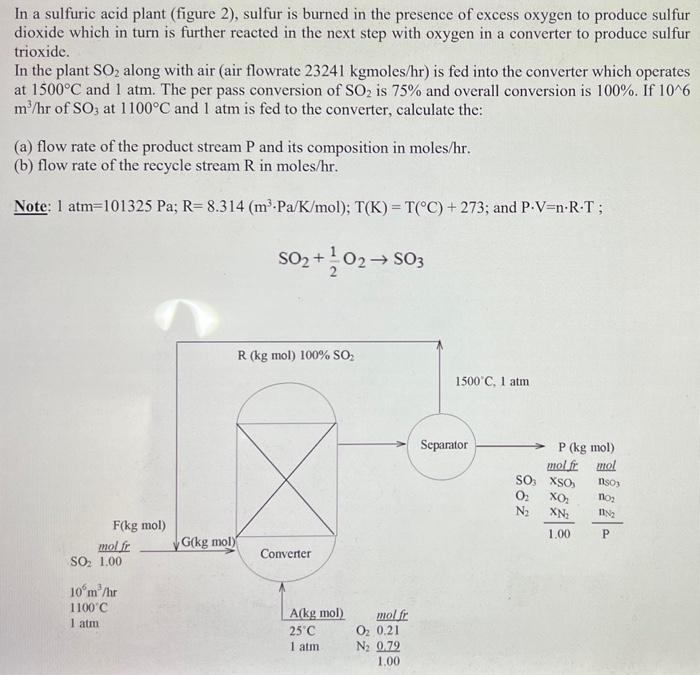
In a sulfuric acid plant (figure 2), sulfur is burned in the presence of excess oxygen to produce sulfur dioxide which in turn is further reacted in the next step with oxygen in a converter to produce sulfur trioxide. In the plant SO2 along with air (air flowrate 23241kgmoles/hr ) is fed into the converter which operates at 1500C and 1atm. The per pass conversion of SO2 is 75% and overall conversion is 100%. If 106 m3/hr of SO3 at 1100C and 1atm is fed to the converter, calculate the: (a) flow rate of the product stream P and its composition in moles/hr. (b) flow rate of the recycle stream R in moles/hr. Note: 1atm=101325Pa;R=8.314(m3Pa/K/mol);T(K)=T(C)+273; and PV=nRT; SO2+21O2SO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


