Question: please solve the first one:) please solve:) What is the standard change in Gibbs free energy for the reaction in the forward direction? ( What
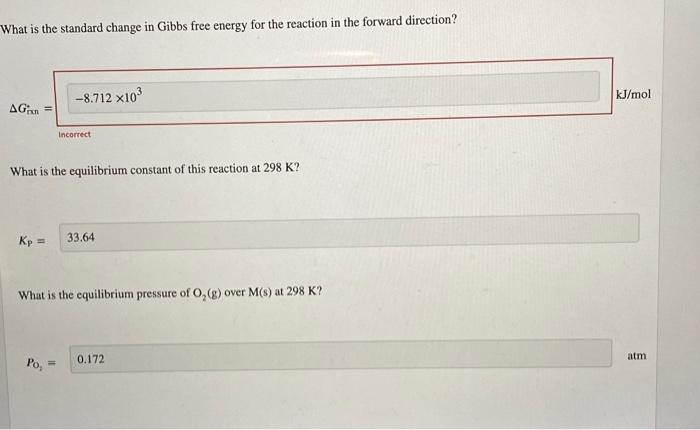
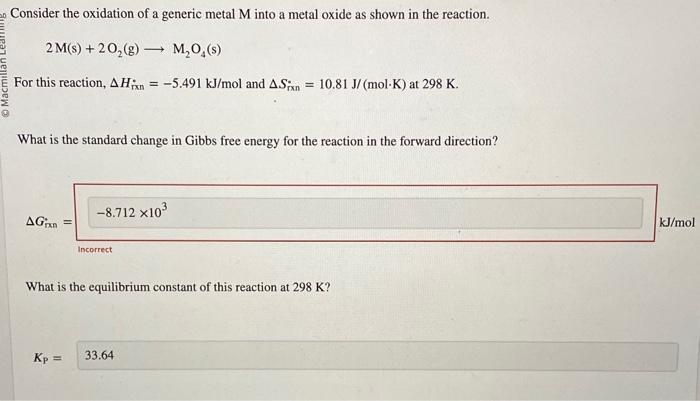
What is the standard change in Gibbs free energy for the reaction in the forward direction? ( What is the equilibrium constant of this reaction at 298K ? K1 What is the equilibrium pressure of O2(g) over M(s) at 298K ? Consider the oxidation of a generic metal M into a metal oxide as shown in the reaction. 2M(s)+2O2(g)M2O4(s) For this reaction, Hrin=5.491kJ/mol and Srxn=10.81J/(molK) at 298K. What is the standard change in Gibbs free energy for the reaction in the forward direction? G Incorrect What is the equilibrium constant of this reaction at 298K ? KP=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


