Question: Please solve the question below and explain it. Thank you. 1. (25 pts) The following liquid-phase reaction sequence is carried out isothermally in a CSTR
Please solve the question below and explain it. Thank you.
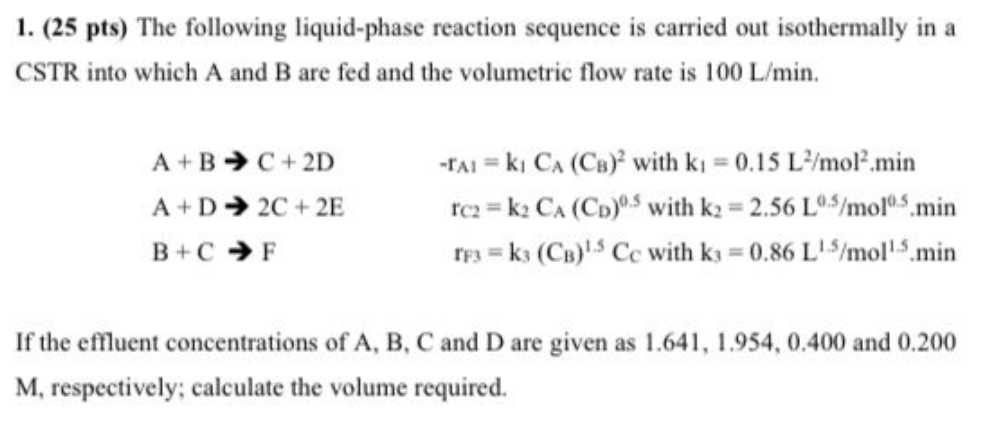
1. (25 pts) The following liquid-phase reaction sequence is carried out isothermally in a CSTR into which A and B are fed and the volumetric flow rate is 100L/min. A+BC+2DrA=k1CA(CB)2withk1=0.15L2/mol2minA+D2C+2ErC2=k2CA(CD)0.5withk2=2.56L0.5/mol0.5minB+CFrF3=k3(CB)1.5CCwithk3=0.86L1.5/mol1.5min If the effluent concentrations of A,B,C and D are given as 1.641,1.954,0.400 and 0.200 M, respectively; calculate the volume required
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


