Question: Please, solve this problem: Using the collected upstream pressures (P1 and P3) and the gas temperature values for both oxidizer and fuel (provided in the
Please, solve this problem:
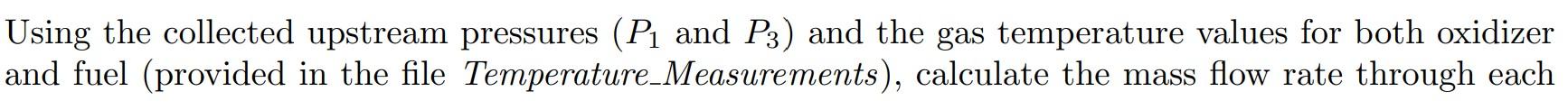
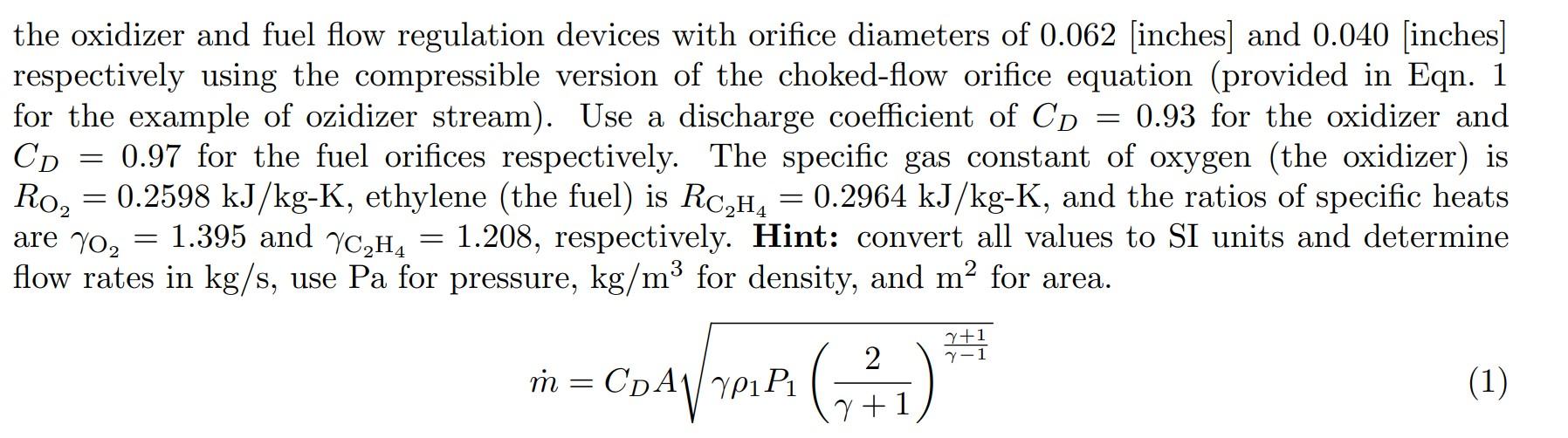
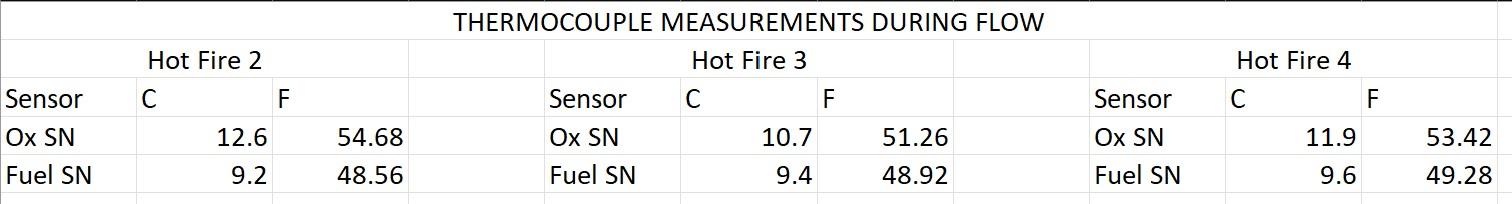
Using the collected upstream pressures (P1 and P3) and the gas temperature values for both oxidizer and fuel (provided in the file Temperature_Measurements), calculate the mass flow rate through each the oxidizer and fuel flow regulation devices with orifice diameters of 0.062 [inches] and 0.040 [inches] respectively using the compressible version of the choked-flow orifice equation (provided in Eqn. 1 for the example of ozidizer stream). Use a discharge coefficient of CD=0.93 for the oxidizer and CD=0.97 for the fuel orifices respectively. The specific gas constant of oxygen (the oxidizer) is RO2=0.2598kJ/kgK, ethylene (the fuel) is RC2H4=0.2964kJ/kgK, and the ratios of specific heats are O2=1.395 and C2H4=1.208, respectively. Hint: convert all values to SI units and determine flow rates in kg /s, use Pa for pressure, kg/m3 for density, and m2 for area. m=CDA1P1(+12)1+1 THERMOCOUPLE MEASUREMENTS DURING FLOW
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


