Question: Please solve this question and show the steps for the graphs Water is frequently disinfected with chlorine gas, forming hypochlorous acid (HOCI), which partially ionizes
 Please solve this question and show the steps for the graphs
Please solve this question and show the steps for the graphs
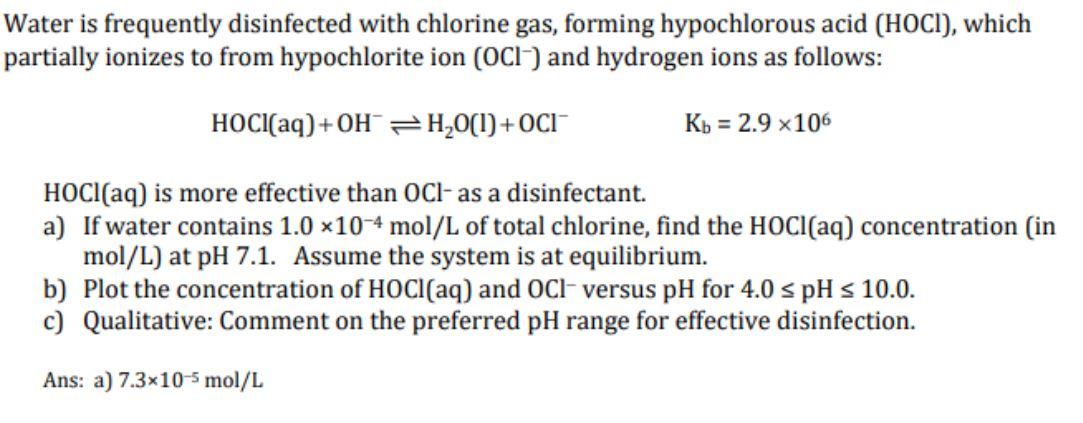
Water is frequently disinfected with chlorine gas, forming hypochlorous acid (HOCI), which partially ionizes to from hypochlorite ion (OCI) and hydrogen ions as follows: HOCl(aq) +OH = H2O(1) OCI Kb = 2.9 x106 HOCI(aq) is more effective than OCl- as a disinfectant. a) If water contains 1.0 *10-4 mol/L of total chlorine, find the HOCl(aq) concentration in mol/L) at pH 7.1. Assume the system is at equilibrium. b) Plot the concentration of HOCl(aq) and OCl- versus pH for 4.0 s pH s 10.0. c) Qualitative: Comment on the preferred pH range for effective disinfection. Ans: a) 7.3x10-5 mol/L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


