Question: PLEASE SOMEONE HELP ME WITH MY ASSIGNMENT, IT IS SUPER HARD FOR ME TO FIGURE IT OUT. THUMBS UP FOR ANYONE WHO GET THE RIGHT
PLEASE SOMEONE HELP ME WITH MY ASSIGNMENT, IT IS SUPER HARD FOR ME TO FIGURE IT OUT. THUMBS UP FOR ANYONE WHO GET THE RIGHT ANSWER AND FIGURES IT OUT.
ASSIGNMENT 2
For the metapelite in your rock and mineral box, list the approximate proportions of each of its constituent minerals. Use these data, the chemical formula of typical specimens of common rock-forming minerals found in metapelites provided and a periodic table to estimate the bulk composition of each specimen. Begin by converting from volume % to weight % of each mineral in each specimen. Then calculate the weight % of each oxide in each specimen. For simplicity, we will work with Mg end members. In the following table, enter the weight % of each of the following minerals which are found in your rock: How do I convert from volume % to weight %? For each specimen, 1) multiply the volume % of each mineral by its density, and 2) divide each value you obtain by the sum of these values for the minerals in the specimen.
| Mineral | Chemical formula | Volume percent (vol. %) | Density (g/cm3) | Weight percent (wt. %) |
| Chlorite* | Mg5Al2Si3O10(OH)8 | 3.0 | ||
| Biotite* | KMg3AlSi3O10(OH)2 | 3.0 | ||
| Garnet* | Mg3Al2Si3O12 | 4.3 | ||
| Staurolite*,** | Mg2Al9Si4O23(OH) | 3.8 | ||
| Kyanite | Al2SiO5 | 3.6 | ||
| Sillimanite | Al2SiO5 | 2.7 | ||
| Muscovite | KAl3Si3O10(OH)2 | 2.8 | ||
| Quartz | SiO2 | 2.7 |
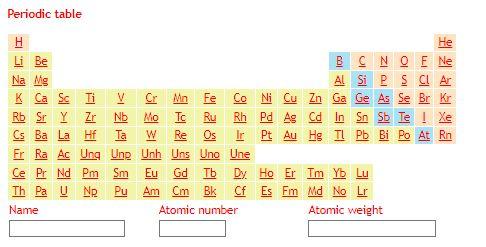
* Chlorite, biotite, garnet and staurolite are shown as Mg end members for simplicity. ** The chemical formula of staurolite has been simplified. In the following table, enter the weight % of each oxide in each of the following minerals which are found in your rock: How do I calculate the weight % of an oxide in a mineral? Begin by calculating the molar weight of each oxide from the molar weights of its constituent atoms (using the following periodic table). Then 1) calculate the molar weight of your mineral from the molar weights of its constituent oxides; 2) calculate the molar weight of each oxide in your mineral by multiplying the molar weight of that oxide by the number of moles of that oxide in your mineral (using chemical formula from the previous table); and 3) divide the molar weight of each oxide in your mineral by the molar weight of the mineral.
| Oxide (wt. %) | Mineral | |||||||
| Chlorite | Biotite | Garnet | Staurolite | Kyanite | Sillimanite | Muscovite | Quartz | |
| SiO2 | ||||||||
| Al2O3 | ||||||||
| MgO | ||||||||
| K2O | ||||||||
| H2O | ||||||||
In the following table, calculate the weight % of each oxide in your rock: How do I calculate the weight % of an oxide in a rock? Multiply the weight % of an oxide in each mineral by the weight % of that mineral in the rock. The sum of these products gives the weight % of the oxide in the rock.
| Oxide (wt. %) | Total |
| SiO2 | |
| Al2O3 | |
| MgO | |
| K2O | |
| H2O |
Periodic table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


