Question: Please step by step Show how to find slope and intersection The reaction A - B is carried out in a laboratory reactor. The concentration
Please step by step
Show how to find slope and intersection
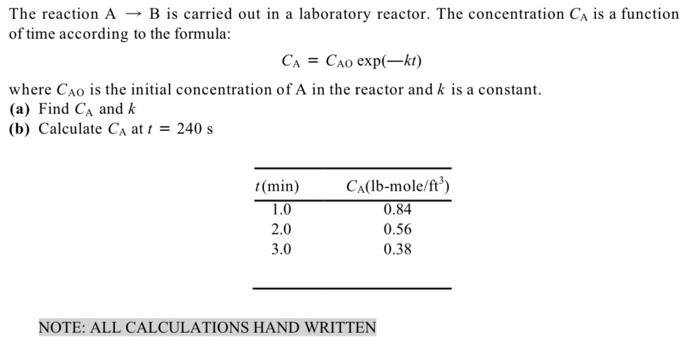
The reaction A - B is carried out in a laboratory reactor. The concentration CA is a function of time according to the formula: CA = CAO exp(-kt) where Cao is the initial concentration of A in the reactor and k is a constant. (a) Find CA and k (b) Calculate CA at 1 = 240 s (min) 1.0 2.0 3.0 Ca(lb-mole/ft?) 0.84 0.56 0.38 NOTE: ALL CALCULATIONS HAND WRITTEN
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


