Question: Please type answer I will thumbs up, thank you! Reaction 1: HNO3(aq)+H2O()H3O+(aq)+NO31(aq)K=24 Reaction 2: NH3(aq)+H2O()NH4+(aq)+OH1(aq)K=1.8105 1. a) Identify the Brnsted-Lowry acid and base for each
Please type answer I will thumbs up, thank you!
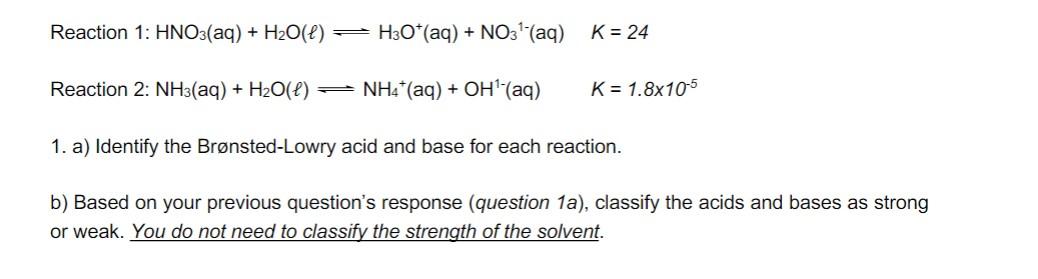
Reaction 1: HNO3(aq)+H2O()H3O+(aq)+NO31(aq)K=24 Reaction 2: NH3(aq)+H2O()NH4+(aq)+OH1(aq)K=1.8105 1. a) Identify the Brnsted-Lowry acid and base for each reaction. b) Based on your previous question's response (question 1a), classify the acids and bases as strong or weak. You do not need to classify the strength of the solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


