Question: Please use a new script (.m) file and save it with your FirstName_ID_Q2. Consider the following reactions for the chlorination of benzene: C6H6+Cl2C6H5Cl+HClC6H5Cl+Cl2C6H4Cl2+HClC6H4Cl2+Cl2C6H3Cl3+HCl The reactor
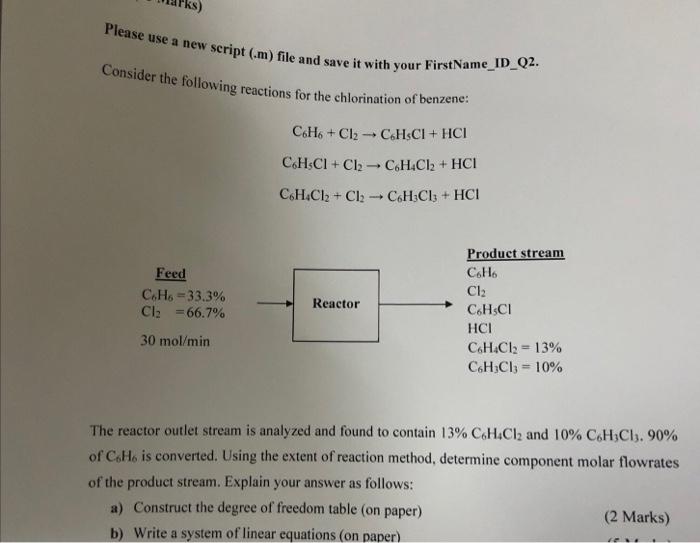
Please use a new script (.m) file and save it with your FirstName_ID_Q2. Consider the following reactions for the chlorination of benzene: C6H6+Cl2C6H5Cl+HClC6H5Cl+Cl2C6H4Cl2+HClC6H4Cl2+Cl2C6H3Cl3+HCl The reactor outlet stream is analyzed and found to contain 13%C6H4Cl2 and 10%C6H3Cl3,90% of C6H6 is converted. Using the extent of reaction method, determine component molar flowrates of the product stream. Explain your answer as follows: a) Construct the degree of freedom table (on paper) b) Write a system of linear equations (on paper)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


