Question: please use the same format help with 1,2 and 3 solution is needed to completely neutralize mL of a solution according to the balanced chemical
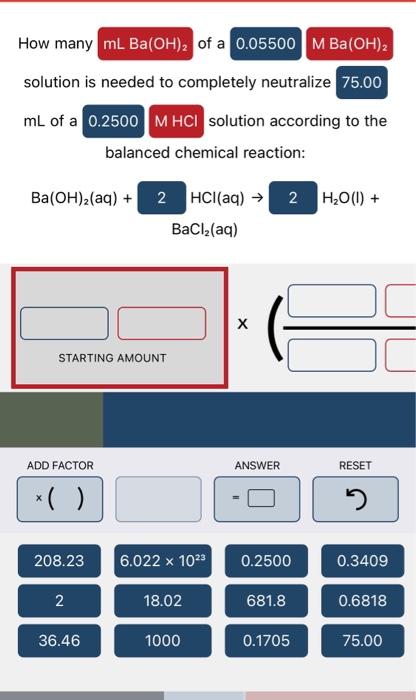
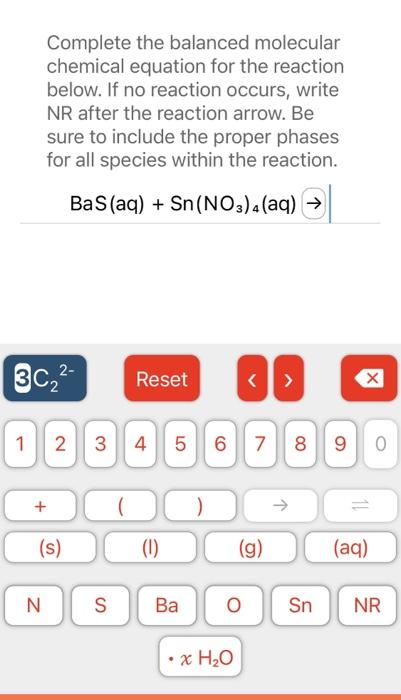
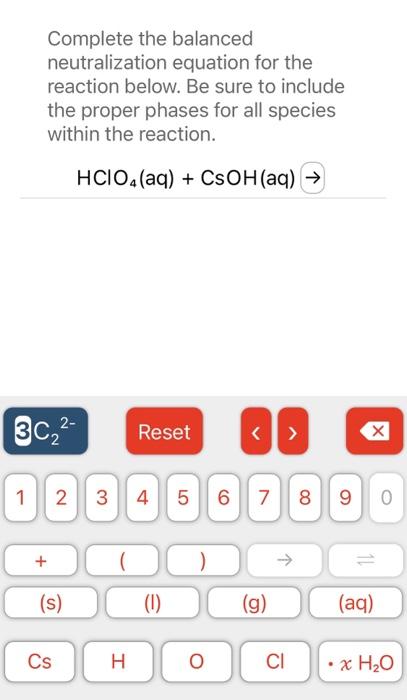
solution is needed to completely neutralize mL of a solution according to the balanced chemical reaction: Ba(OH)2(aq)+HCl(aq)H2O(I)+BaCl2(aq) STARTING AMOUNT Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. BaS(aq)+Sn(NO3)4(aq) Complete the balanced neutralization equation for the reaction below. Be sure to include the proper phases for all species within the reaction. HClO4(aq)+CsOH(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


