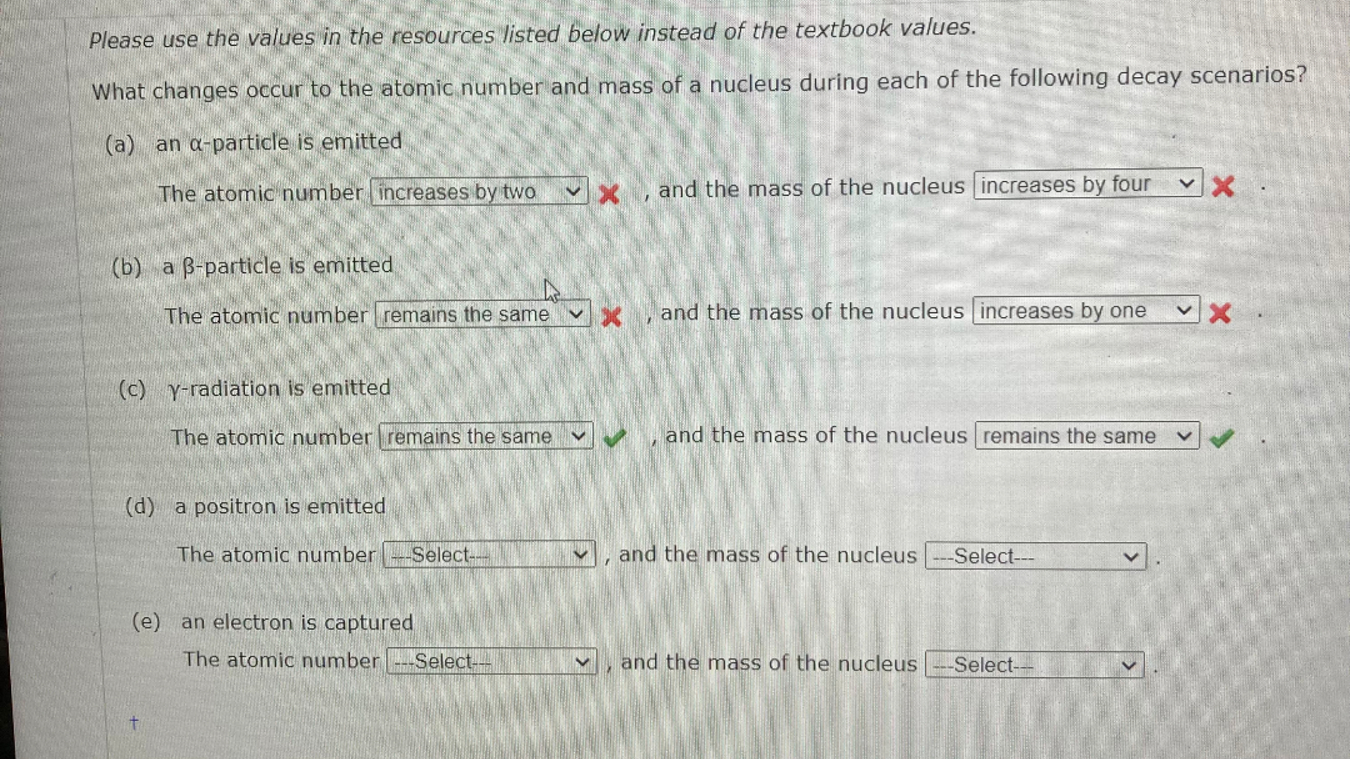
Question: Please use the values in the resources listed below instead of the textbook values. What changes occur to the atomic number and mass of a
Please use the values in the resources listed below instead of the textbook values.
What changes occur to the atomic number and mass of a nucleus during each of the following decay scenarios?
a an particle is emitted
The atomic number increases by two and the mass of the nucleus increases by four
b a particle is emitted
The atomic number remains the same and the mass of the nucleus increases by one
cradiation is emitted
The atomic number
and the mass of the nucleus
d a positron is emitted
The atomic number
and the mass of the nucleus
e an electron is captured
The atomic number
and the mass of the nucleus
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


