Question: Please use the values in the resources listed below instead of the textbook values. Under certain conditions the decomposition of ammonia on a metal surface
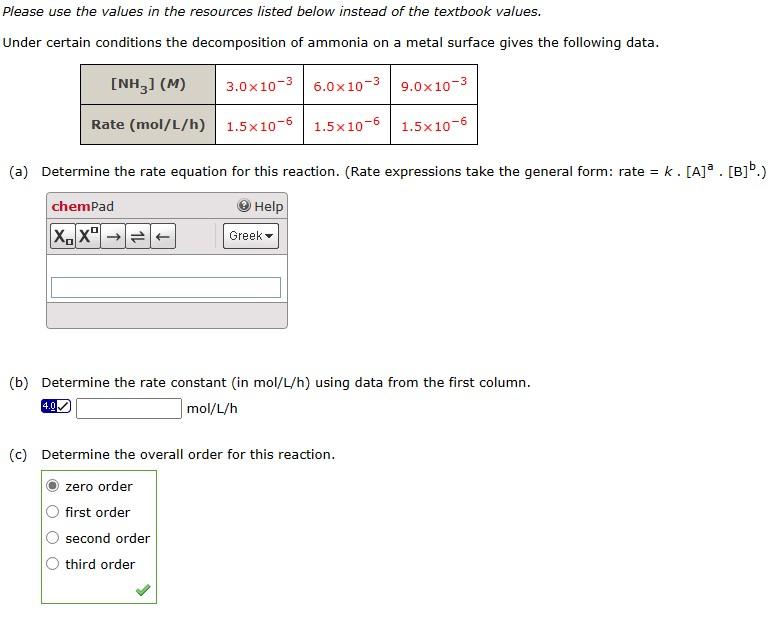
Please use the values in the resources listed below instead of the textbook values. Under certain conditions the decomposition of ammonia on a metal surface gives the following data. (a) Determine the rate equation for this reaction. (Rate expressions take the general form: rate =k[A]a[B]b.) (b) Determine the rate constant (in mol/L/h ) using data from the first column. mol/L/h (c) Determine the overall order for this reaction. zero order first order second order third order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


