Question: Please use the values in the resources listed below instead of the textbook values. Calculate G (in kJ/mol) for each of the following reactions from
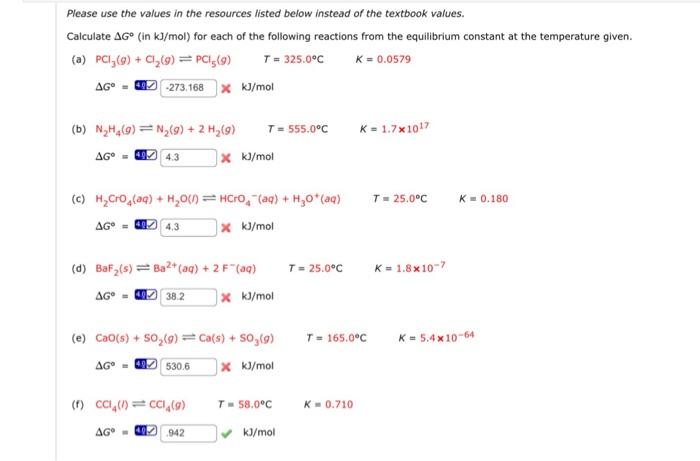
Please use the values in the resources listed below instead of the textbook values. Calculate G (in kJ/mol) for each of the following reactions from the equilibrium constant at the temperature given. (a) PCl3(g)+Cl2(g)PCl5(g)T=325.0CK=0.0579G=40.kJ/mol (b) N2H4(g)N2(g)+2H2(g)T=555.0CK=1.71017G=4.CkJ/mol (c) H2CrO4(aq)+H2O()HCrO4(aq)+H3O+(aq)T=25.0CK=0.180G=4AgkJ/mol (d) BaF2(s)Ba2+(aq)+2F(aq)T=25.0CK=1.8107 G=agkJ/mol (e) CaO(s)+SO2(g)Ca(s)+SO3(g)T=165.0CK=5.41064 G=4gkJ/mol (f) CCl4(h)CCl4(g)T=58.0CK=0.710
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


