Question: Please use the values in the resources listed below instead of the textbook values Tripling the concentration of a reactant increases the rate of a
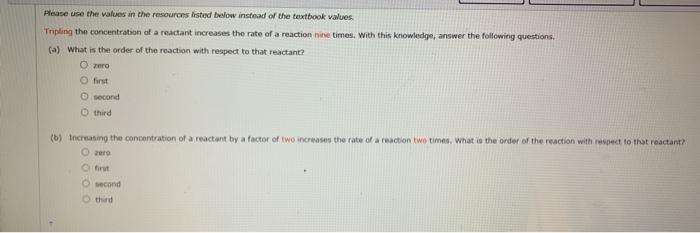
Please use the values in the resources listed below instead of the textbook values Tripling the concentration of a reactant increases the rate of a reaction nine times. With this knowledge, answer the following questions. (a) What is the order of the reaction with respect to that reactant? 0 first second third (6) Increasing the concentration of a reactant by a factor of two increases the rate of a reaction two times. What is the order of the reaction with respect to that reactant second third
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


