Question: Please write the answer in details .Thanks a lot . It has been confirmed that the superb bactericidal activity of silver nanoparticles (AgNPs) comes from
Please write the answer in details .Thanks a lot .
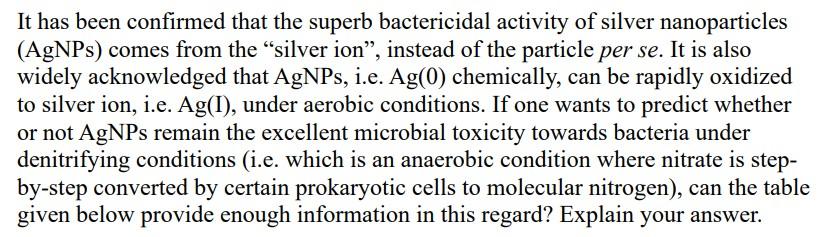
It has been confirmed that the superb bactericidal activity of silver nanoparticles (AgNPs) comes from the silver ion, instead of the particle per se. It is also widely acknowledged that AgNPs, i.e. Ag(0) chemically, can be rapidly oxidized to silver ion, i.e. Ag(I), under aerobic conditions. If one wants to predict whether or not AgNPs remain the excellent microbial toxicity towards bacteria under denitrifying conditions (i.e. which is an anaerobic condition where nitrate is step- by-step converted by certain prokaryotic cells to molecular nitrogen), can the table given below provide enough information in this regard? Explain your answer. - Redox Reactions Ag(0) + 1/5 NO3 + 6/5 H* = Ag(I) + 1/10 N2(aq) + 3/5 H2O Ag(0)s) + 1/3 NO2 + 4/3 H* = Ag(I) + 1/6 N2(aq) + 2/3 H2O Ag(0)s) + 1/2 NO(aq) + H+ = Ag(I) + 1/4 N2(aq) + 1/2 H20 Ag(Os) + 1/2 N2O(aq) + H*= Ag(1) + 1/2 N2(aq) + 1/2 H20 AG (kJ mol!) - 41.22 - 66.25 - 88.2 -- -- -- - 87.57
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


