Question: PLEASE WRITE THE GIVEN, REQUIRED, FORMULA, SOLUTION, AND ANSWER FOR EACH PROBLEM. DIRECTION: Solve for what is asked in the given problems below about Specific
PLEASE WRITE THE GIVEN, REQUIRED, FORMULA, SOLUTION, AND ANSWER FOR EACH PROBLEM.
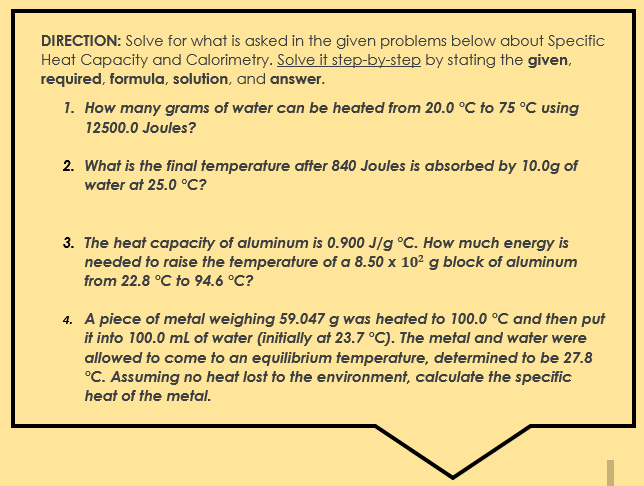
DIRECTION: Solve for what is asked in the given problems below about Specific Heat Capacity and Calorimetry. Solve it step-by-step by stating the given, required, formula, solution, and answer. 1. How many grams of water can be heated from 20.0 C to 75 "C using 12500.0 Joules? 2. What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0 .C? 3. The heat capacity of aluminum is 0.900 J/g "C. How much energy is needed to raise the temperature of a 8.50 x 102 g block of aluminum from 22.8 "C to 94.6 "C? 4. A piece of metal weighing 59.047 g was heated to 100.0 C and then put it into 100.0 ml of water (initially at 23.7 *C). The metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 "C. Assuming no heat lost to the environment, calculate the specific heat of the metal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


