Question: Pleaseeeeee help. i need explanations and details for the both of them. Problem 1 (100 points): A liquid bioreaction A B+C has a reaction rate
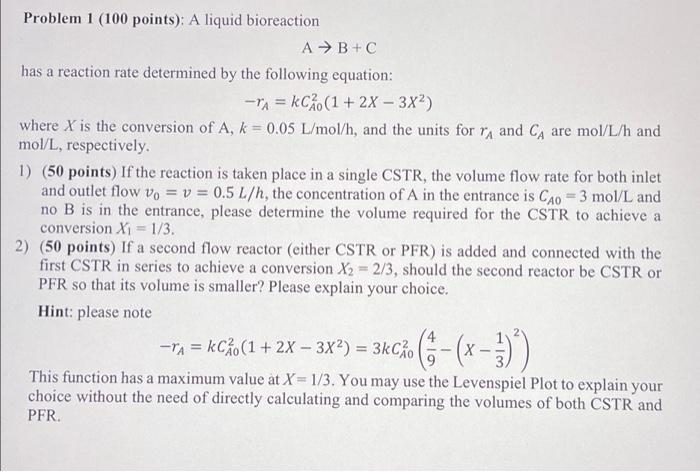
Problem 1 (100 points): A liquid bioreaction A B+C has a reaction rate determined by the following equation: -A = {Co(1+2X 3x2) where X is the conversion of A, k = 0.05 L/mol/h, and the units for rA and CA are mol/L/h and mol/L, respectively. 1) (50 points) If the reaction is taken place in a single CSTR, the volume flow rate for both inlet and outlet flow vo = v = 0.5 L/h, the concentration of A in the entrance is CAO = 3 mol/L and no B is in the entrance, please determine the volume required for the CSTR to achieve a conversion Xi = 1/3 2) (50 points) If a second flow reactor (either CSTR or PFR) is added and connected with the first CSTR in series to achieve a conversion X2 = 2/3, should the second reactor be CSTR or PFR so that its volume is smaller? Please explain your choice. Hint: please note = kcko(1+2X 3X2) = 3kco (6 (x-3)) X This function has a maximum value at X = 1/3. You may use the Levenspiel Plot to explain your choice without the need of directly calculating and comparing the volumes of both CSTR and PFR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


