Question: Plot the graph of your data collected from the distillation. On the graph, show which fractions will be used to calculate the percentage of cyclohexane,
Plot the graph of your data collected from the distillation. On the graph, show which fractions will be used to calculate the percentage of cyclohexane, which fractions will be used to calculate the percentage of toluene, and which fractions are a mixture of the two. Take all mixtures as 50% cyclohexane and 50% toluene. The liquid remaining in the distillation flask must be included.
You need to plot a graph for the fractional distillation. You can use Excel, Logger Pro or the preferred software. Make sure your graph has a title (which reads Fig. SD.3), well-labeled axes, units where needed, an appropriate scale, indicated values for volumes, and a line connecting them. As described in the instructions, label the regions that correspond to each liquid or mixture.
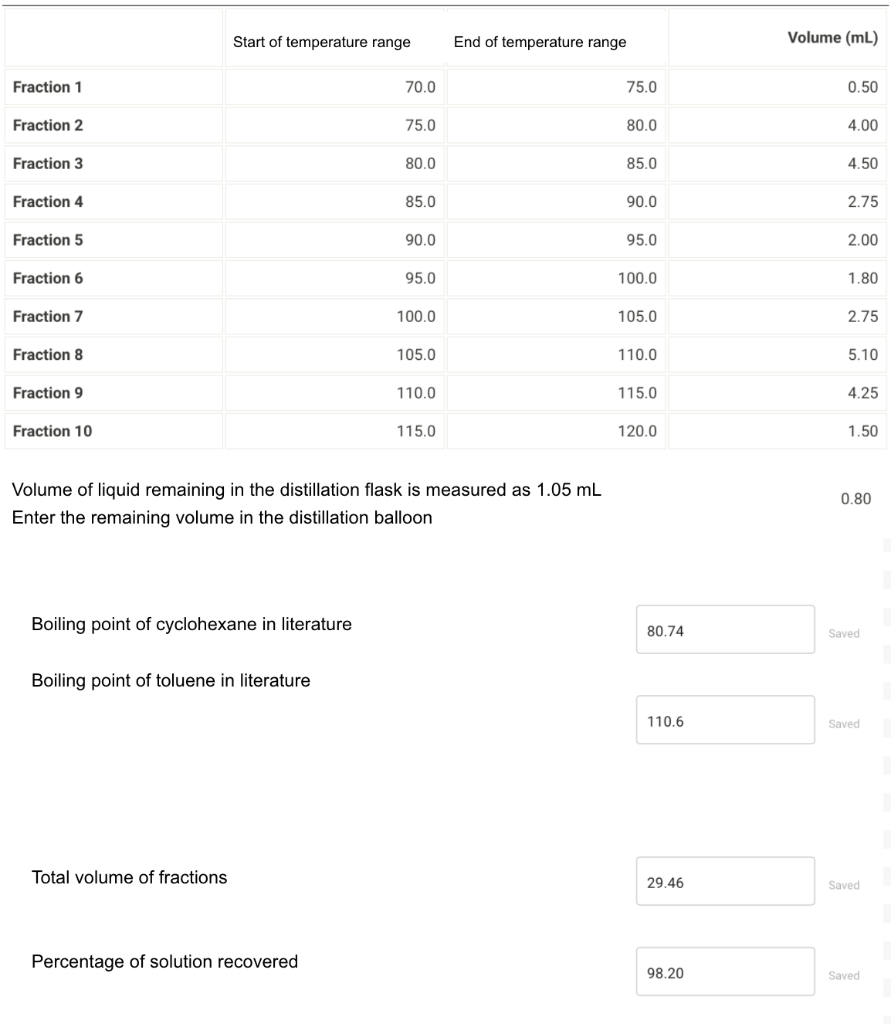
Start of temperature range End of temperature range Volume (mL) Fraction 1 70.0 75.0 0.50 Fraction 2 75.0 80.0 4.00 Fraction 3 80.0 85.0 4.50 Fraction 4 85.0 90.0 2.75 Fraction 5 90.0 95.0 2.00 Fraction 6 95.0 100.0 1.80 Fraction 7 100.0 105.0 2.75 Fraction 8 105.0 110.0 5.10 Fraction 9 110.0 115.0 4.25 Fraction 10 115.0 120.0 1.50 0.80 Volume of liquid remaining in the distillation flask is measured as 1.05 mL Enter the remaining volume in the distillation balloon Boiling point of cyclohexane in literature 80.74 Saved Boiling point of toluene in literature 110.6 Saved Total volume of fractions 29.46 Saved Percentage of solution recovered 98.20 Saved Start of temperature range End of temperature range Volume (mL) Fraction 1 70.0 75.0 0.50 Fraction 2 75.0 80.0 4.00 Fraction 3 80.0 85.0 4.50 Fraction 4 85.0 90.0 2.75 Fraction 5 90.0 95.0 2.00 Fraction 6 95.0 100.0 1.80 Fraction 7 100.0 105.0 2.75 Fraction 8 105.0 110.0 5.10 Fraction 9 110.0 115.0 4.25 Fraction 10 115.0 120.0 1.50 0.80 Volume of liquid remaining in the distillation flask is measured as 1.05 mL Enter the remaining volume in the distillation balloon Boiling point of cyclohexane in literature 80.74 Saved Boiling point of toluene in literature 110.6 Saved Total volume of fractions 29.46 Saved Percentage of solution recovered 98.20 Saved
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


