Question: Pls complete the enthalpy table, and solve for the rate of hear transfer in kW. Thanks Ammonia is oxidized with air to form nitric oxide
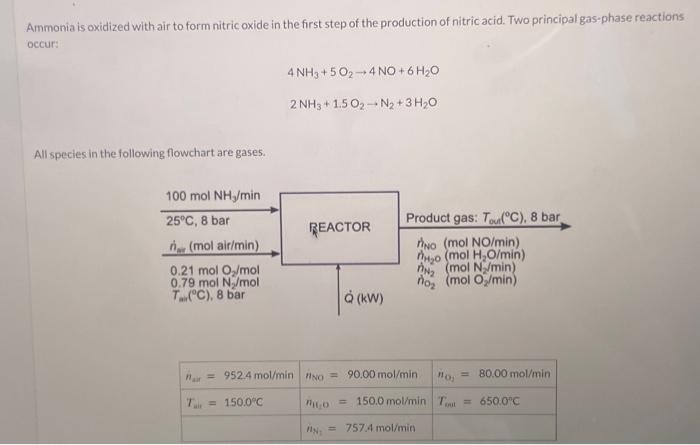
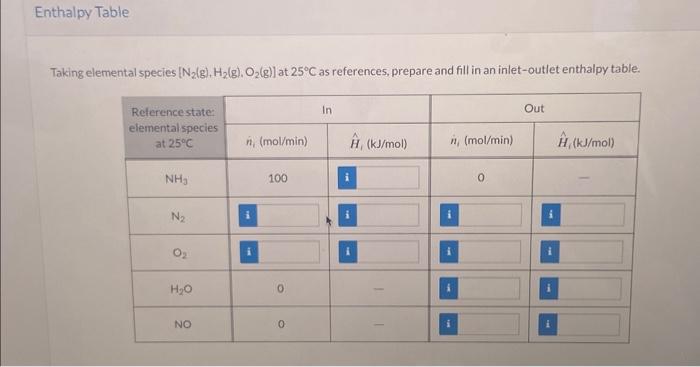
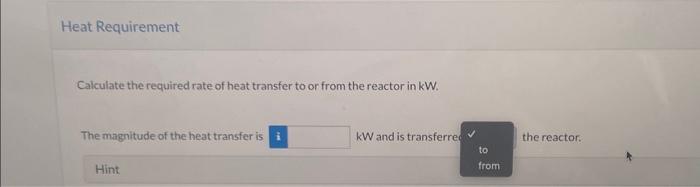
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal gas-phase reactions occur: 4NH3+5O24NO+6H2O2NH3+1.5O2N2+3H2O All spectes in the following flowchart are gases. Taking elemental species [N2(g),H2(g),O2(g)] at 25C as references, prepare and fill in an inlet-outlet enthalpy table. Calculate the required rate of heat transfer to or from the reactor in kW. The magnitude of the heat transfer is kW and is transferre the reactor. Hint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


