Question: second time asking, could really use the help. please include the required rate of heat transfer to/frin the reactor in kW (use enthalpy table) Ammonia
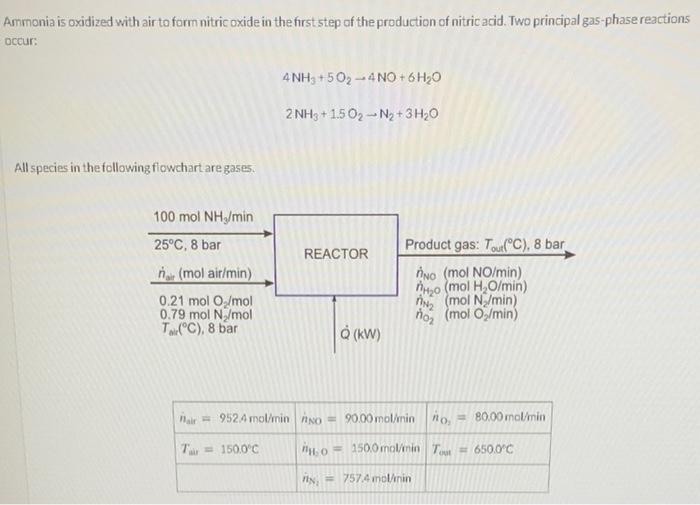
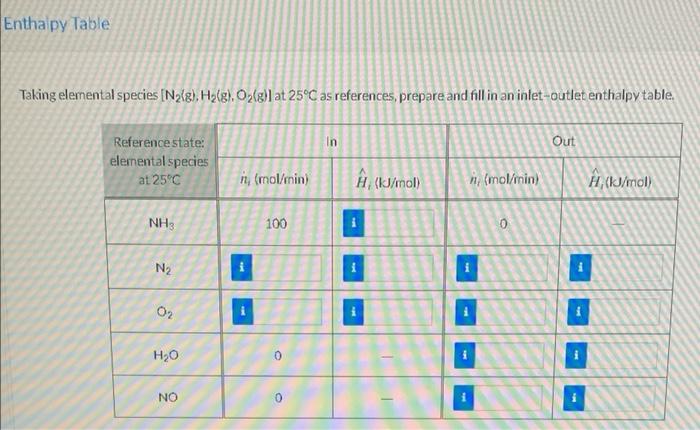
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal gas-phase reactions occur 4 NH3 + 50, - 4 NO + 6H30 2 NH3 + 1.5 02-N2 + 3H20 All species in the following fiowchart are gases. REACTOR 100 mol NH /min 25C, 8 bar nor (mol air/min) 0.21 mol O /mol 0.79 mol N/mol T. (C), 8 bar Product gas: ToulC), 8 bar NO (mol NO/min) Ho (mol H2O/min) (mol N/min) (mol O/min) MINE noz 0 (kW) Mair = 952 A mol/min NO = 90.00 mol/vino, = 80.00 mol/min 7 = 150.0C = 1500 mol/min T 650.0C = 7574 mol/min Entha py Table Taking elemental species [Nalg), H2(g), O2(g)) at 25C as references, prepare and fill in an intet-outlet enthalpy table. In Out Reference state: elemental species at 25C ni (mol/min) (kJ/mol n (mol/min) # (kJ/mol NH3 100 0 N2 O2 i H20 0 NO 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


