Question: Pls help!! Given the structure below, which would have longer bond length? b would have longer bond length same bond length None of these a
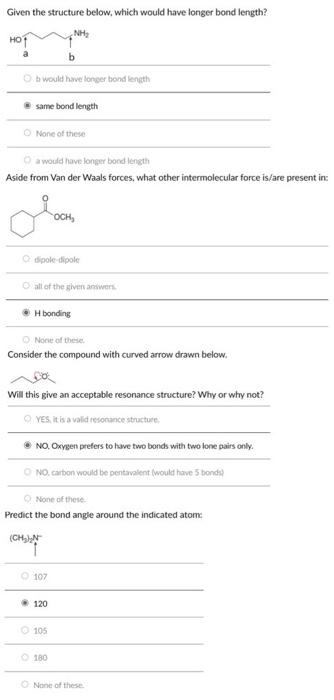
Given the structure below, which would have longer bond length? b would have longer bond length same bond length None of these a would have longer bocd length Aside from Van der Waals forces, what other intermolecular force is/are present in: tipole-dipole all of the given anowern. H bonding None of these. Consider the compound with curved arrow drawn below. s Will this give an acceptable resonance structure? Why or why not? Yes, it is a valid reforance structure. NO, Oxygen prefers to have two bonds with two lone pairs only. No, carbon would be pentavilent (would have 5 bonds? None of these. Predict the bond angle around the indicated atome None of these
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


