Question: Pls help me solve 1 and 2 Solubility is a quantitative term. It is defined as the maximum amount of solute that will dissolve in
Pls help me solve 1 and 2
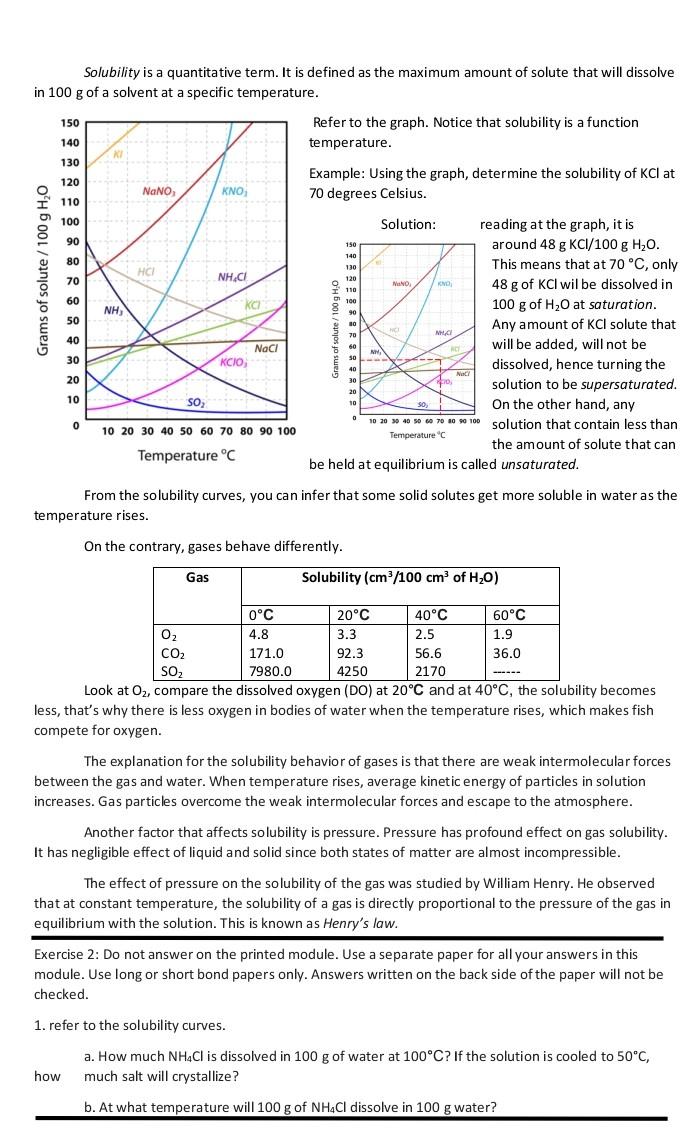
Solubility is a quantitative term. It is defined as the maximum amount of solute that will dissolve in 100 g of a solvent at a specific temperature. Refer to the graph. Notice that solubility is a function temperature. 150 140 130 120 NaNO Example: Using the graph, determine the solubility of KCl at 70 degrees Celsius. KNO, 110 100 90 80 150 140 130 Grams of solute / 100 g H,0 XHC 70 NH.CH NANO KO 120 I 110 O 100 8 90 60 ka , 50 10 M 40 Naci Solution: reading at the graph, it is around 48 g KCl/100 g H2O. This means that at 70 C, only 48 g of KCl wil be dissolved in 100 g of H2O at saturation. Any amount of KCl solute that will be added, will not be dissolved, hence turning the solution to be supersaturated. On the other hand, any 10 20 40 50 60 70 100 100 solution that contain less than the amount of solute that can be held at equilibrium is called unsaturated. 70 60 30 NH, 30 KCIO, 40 30 Na 20 10 20 10 SO 0 Temperature" 10 20 30 40 50 60 70 80 90 100 Temperature C From the solubility curves, you can infer that some solid solutes get more soluble in water as the temperature rises. On the contrary, gases behave differently. Gas Solubility (cm/100 cm of H20) 0C 20C 40C 60C 02 4.8 3.3 2.5 1.9 CO2 171.0 92.3 56.6 36.0 SOZ 7980.0 4250 2170 Look at O2, compare the dissolved oxygen (DO) at 20C and at 40C, the solubility becomes less, that's why there is less oxygen in bodies of water when the temperature rises, which makes fish compete for oxygen. The explanation for the solubility behavior of gases is that there are weak intermolecular forces between the gas and water. When temperature rises, average kinetic energy of particles in solution increases. Gas particles overcome the weak intermolecular forces and escape to the atmosphere. Another factor that affects solubility is pressure. Pressure has profound effect on gas solubility. It has negligible effect of liquid and solid since both states of matter are almost incompressible. The effect of pressure on the solubility of the gas was studied by William Henry. He observed that at constant temperature, the solubility of a gas is directly proportional to the pressure of the gas in equilibrium with the solution. This is known as Henry's law. Exercise 2: Do not answer on the printed module. Use a separate paper for all your answers in this module. Use long or short bond papers only. Answers written on the back side of the paper will not be checked. 1. refer to the solubility curves. a. How much NH4Cl is dissolved in 100 g of water at 100C? If the solution is cooled to 50C, much salt will crystallize? how b. At what temperature will 100 g of NH4Cl dissolve in 100 g water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


