Question: pls help On the following structure (assuming octets for all atoms other than H ): a. Draw in any nonbonding valence electrons that are missing
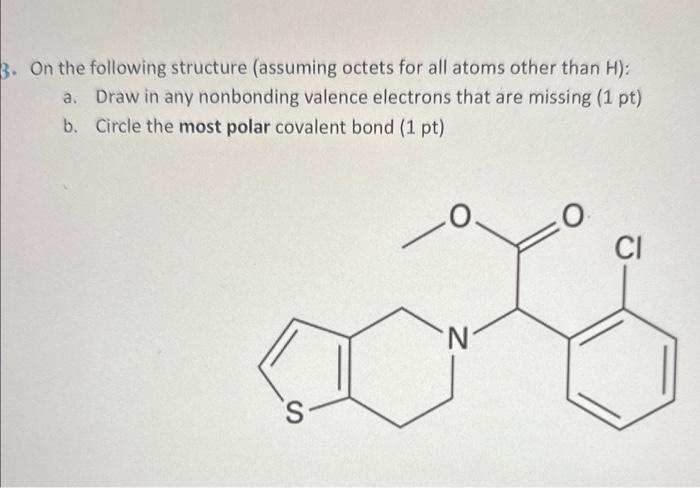
On the following structure (assuming octets for all atoms other than H ): a. Draw in any nonbonding valence electrons that are missing (1 pt) b. Circle the most polar covalent bond ( 1pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


