Question: 1. Summarize: The principal quantum number, n, can have the values of: The angular momentum quantum number, I, can have integer values from The
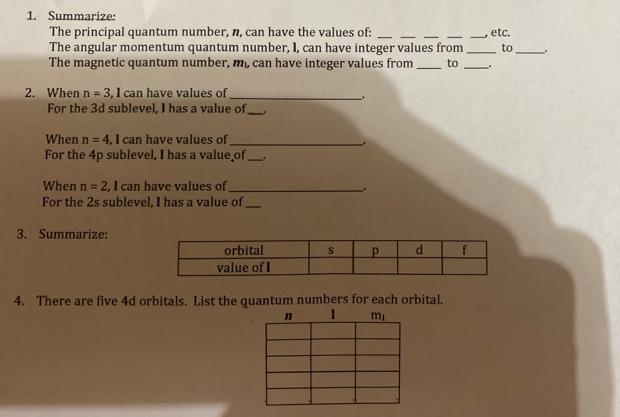
1. Summarize: The principal quantum number, n, can have the values of: The angular momentum quantum number, I, can have integer values from The magnetic quantum number, m, can have integer values from to 2. When n = 3,1 can have values of For the 3d sublevel, I has a value of When n = 4, I can have values of For the 4p sublevel, I has a value of When n = 2,1 can have values of For the 2s sublevel, I has a value of 3. Summarize: orbital value of I S n d 4. There are five 4d orbitals. List the quantum numbers for each orbital. 1 mi f etc. to 1. Summarize: The principal quantum number, n, can have the values of: The angular momentum quantum number, I, can have integer values from The magnetic quantum number, m, can have integer values from to 2. When n = 3,1 can have values of For the 3d sublevel, I has a value of When n = 4, I can have values of For the 4p sublevel, I has a value of When n = 2,1 can have values of For the 2s sublevel, I has a value of 3. Summarize: orbital value of I S n d 4. There are five 4d orbitals. List the quantum numbers for each orbital. 1 mi f etc. to
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
1Principle quantom number denoted by the letter n is the main energy level shell in which electrons ... View full answer

Get step-by-step solutions from verified subject matter experts


