Question: pls write step by step A cylindrical graphite (pure carbon) rod of length 25cm and initial diameter of 2cm is surrounded by air at 1100K
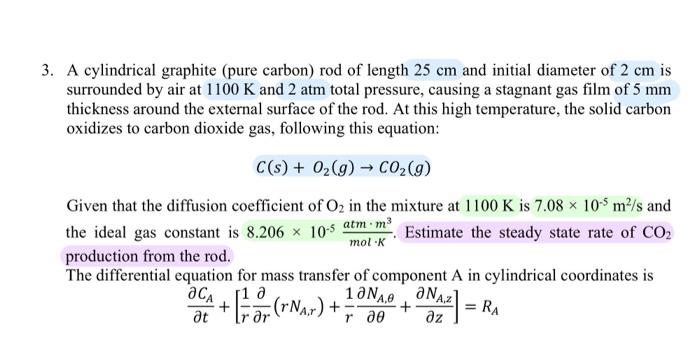
A cylindrical graphite (pure carbon) rod of length 25cm and initial diameter of 2cm is surrounded by air at 1100K and 2 atm total pressure, causing a stagnant gas film of 5mm thickness around the external surface of the rod. At this high temperature, the solid carbon oxidizes to carbon dioxide gas, following this equation: C(s)+O2(g)CO2(g) Given that the diffusion coefficient of O2 in the mixture at 1100K is 7.08105m2/s and the ideal gas constant is 8.206105molKatmm3. Estimate the steady state rate of CO2 production from the rod. The differential equation for mass transfer of component A in cylindrical coordinates is tCA+[r1r(rNA,r)+r1NA,+zNA,z]=RA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


