Question: A process stream, 150 kg / h containing 40 mass% ethanol (C2H6O), 15 mass% methanol (CH4O) and the rest water is fed to a separation
A process stream, 150 kg / h containing 40 mass% ethanol (C2H6O), 15 mass% methanol (CH4O) and the rest water is fed to a separation plant, according to the figure below. After the separation, two product streams are taken out, one with a flow of 60 kg / h which is in the vapor phase and containing 75 mass% ethanol, 20 mass% methanol and 5 mass% water. The other the flow out of the separator is in the liquid phase and has an unknown composition. Calculate the composition of the three components of the unknown current. The answer should be is given in both mass% and mol%.
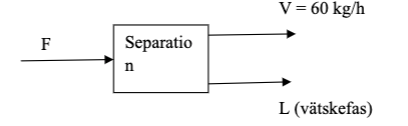 Vtskefas = liquid phase
Vtskefas = liquid phase
V = 60 kg/h F Separatio n L (vtskefas)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


