Question: PLSS STEP BY STEP SOLUTION! :) The first chemical step in making sulfuric acid from elemental sulfur is burning the sulfur (completely) to form SO2.
PLSS STEP BY STEP SOLUTION! :)
The first chemical step in making sulfuric acid from elemental sulfur is burning the sulfur (completely) to form SO2. Calculate the temperature of the gas leaving the burner based on the following: the burner operates adiabatically, sulfur enters as liquid at 140C, and excess air (79% mol N2, 21% O2) enters at 25C; H (S(), 140C S(s,rh), 25C) = -5.07 kJmol-1; the outlet gas contains 9.5 mol % SO2; other data required are given in table.
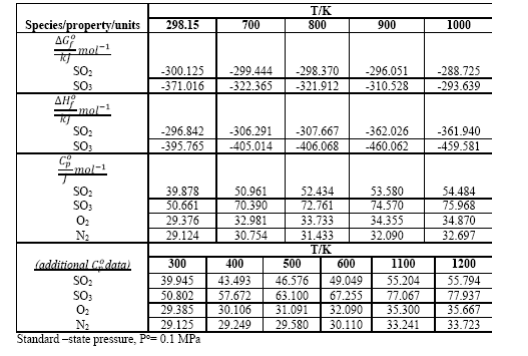
Standard -state pressure, Po=0.1MPa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


