Question: plz help with all or any for a thumbs up = = = A counter flowing heat exchanger (s. Fig. E) for cooling a hot
 plz help with all or any for a thumbs up
plz help with all or any for a thumbs up
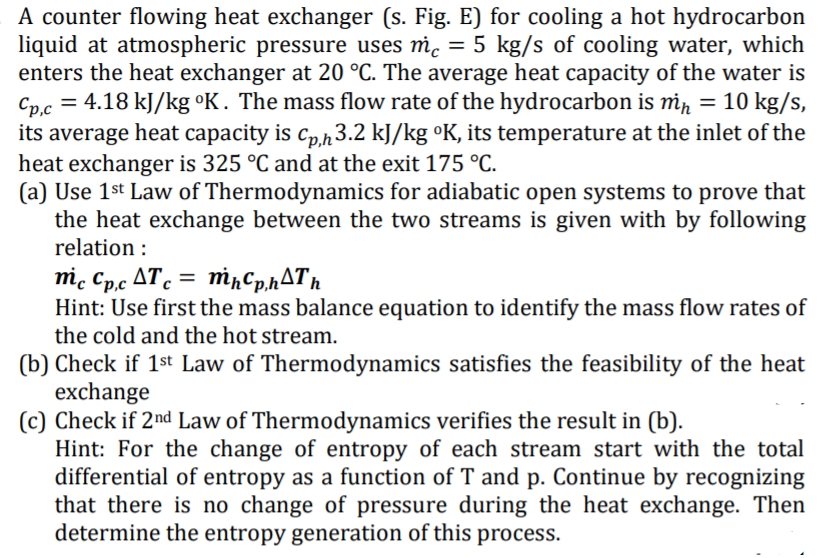
= = = A counter flowing heat exchanger (s. Fig. E) for cooling a hot hydrocarbon liquid at atmospheric pressure uses mc = 5 kg/s of cooling water, which enters the heat exchanger at 20 C. The average heat capacity of the water is Cp,c = 4.18 kJ/kg K. The mass flow rate of the hydrocarbon is min = 10 kg/s, its average heat capacity is Cp,h3.2 kJ/kg K, its temperature at the inlet of the heat exchanger is 325 C and at the exit 175 C. (a) Use 1st Law of Thermodynamics for adiabatic open systems to prove that the heat exchange between the two streams is given with by following relation : mic Cp,c AT, = mnCp,h4Tn Hint: Use first the mass balance equation to identify the mass flow rates of the cold and the hot stream. (b) Check if 1st Law of Thermodynamics satisfies the feasibility of the heat exchange (C) Check if 2nd Law of Thermodynamics verifies the result in (b). Hint: For the change of entropy of each stream start with the total differential of entropy as a function of T and p. Continue by recognizing that there is no change of pressure during the heat exchange. Then determine the entropy generation of this process. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


