Question: potential is: =+RTln(p/p) (a) Derive the following expression for the Gibbs energy of mixing (at constant T and p ) of two perfect gases (A
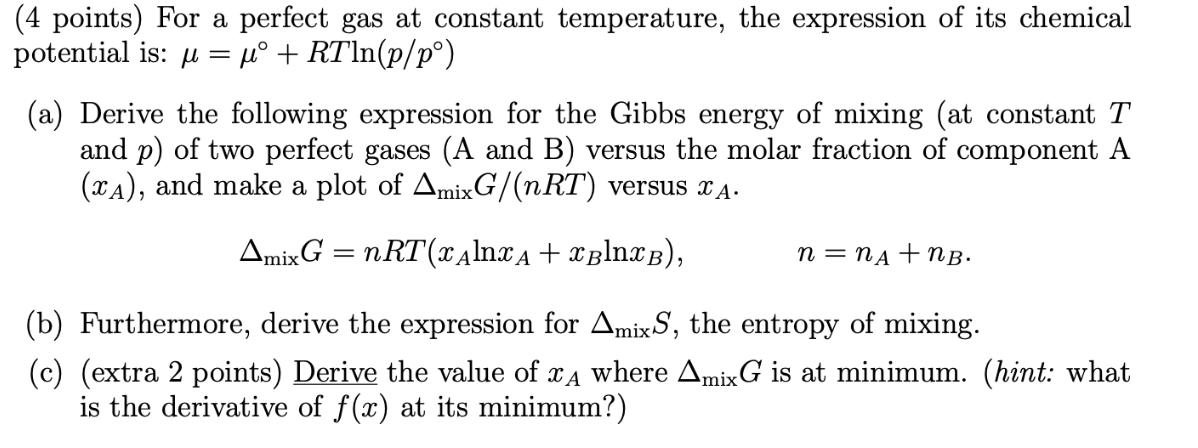
potential is: =+RTln(p/p) (a) Derive the following expression for the Gibbs energy of mixing (at constant T and p ) of two perfect gases (A and B ) versus the molar fraction of component A (xA), and make a plot of mixG/(nRT) versus xA. mixG=nRT(xAlnxA+xBlnxB),n=nA+nB. (b) Furthermore, derive the expression for mixS, the entropy of mixing. (c) (extra 2 points) Derive the value of xA where mixG is at minimum. (hint: what is the derivative of f(x) at its minimum?) potential is: =+RTln(p/p) (a) Derive the following expression for the Gibbs energy of mixing (at constant T and p ) of two perfect gases (A and B ) versus the molar fraction of component A (xA), and make a plot of mixG/(nRT) versus xA. mixG=nRT(xAlnxA+xBlnxB),n=nA+nB. (b) Furthermore, derive the expression for mixS, the entropy of mixing. (c) (extra 2 points) Derive the value of xA where mixG is at minimum. (hint: what is the derivative of f(x) at its minimum?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


