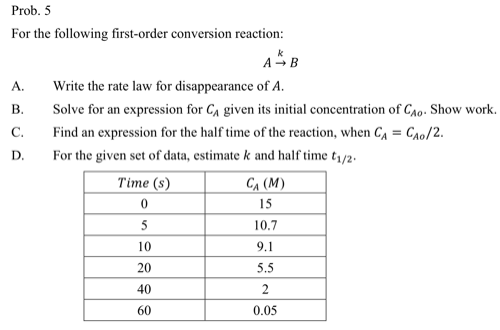
Question: Prob. 5 For the following first - order conversion reaction: A k B A . Write the rate law for disappearance of A . B
Prob.
For the following firstorder conversion reaction:
A Write the rate law for disappearance of
B Solve for an expression for given its initial concentration of Show work.
C Find an expression for the half time of the reaction, when
D For the given set of data, estimate and half time
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


