Question: Problem 1: (1) Write down the reaction rate for and production rate for C, assuming the reaction follow an elementary rate law. (10 points) 24+*+
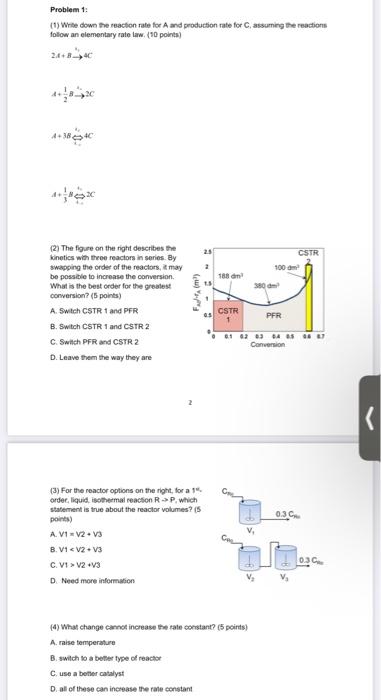
Problem 1: (1) Write down the reaction rate for and production rate for C, assuming the reaction follow an elementary rate law. (10 points) 24+*+ 4+38*** 25 CSTR 100 m 2 380 (2) The figure on the right describes the kinetics with the reactors in series By swapping the order of the reactors, may be possible to increase the conversion What is the best order for the greatest conversion? (5 points) A. Switch CSTR 1 and PFR B. Switch CSTR 1 and CSTR 2 C Switch PFR and CSTR 2 D. Leave them the way they are CSTR PFR 0 01 02 03 04 05 08 1 Conversion 0.3 v, (3) For the reactor options on the right for a order liquid thermal reaction RP which statement is true about the reactor volumes? (5 points) A.V1 V2 V3 8. V1 V2 V3 C.VI>V2 V3 D. Need more information Cou 030 V V {} What change cannot increase the rate constant? (5 points) A raise temperature B. switch to a better type of reactor C. use a better catalyst D. all of these can increase the rate constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


