Question: Problem 1; 60 points) A tower, 15 cm. in diameter, is to be used to lower the ammonia (NH3) concentration in a gas stream from
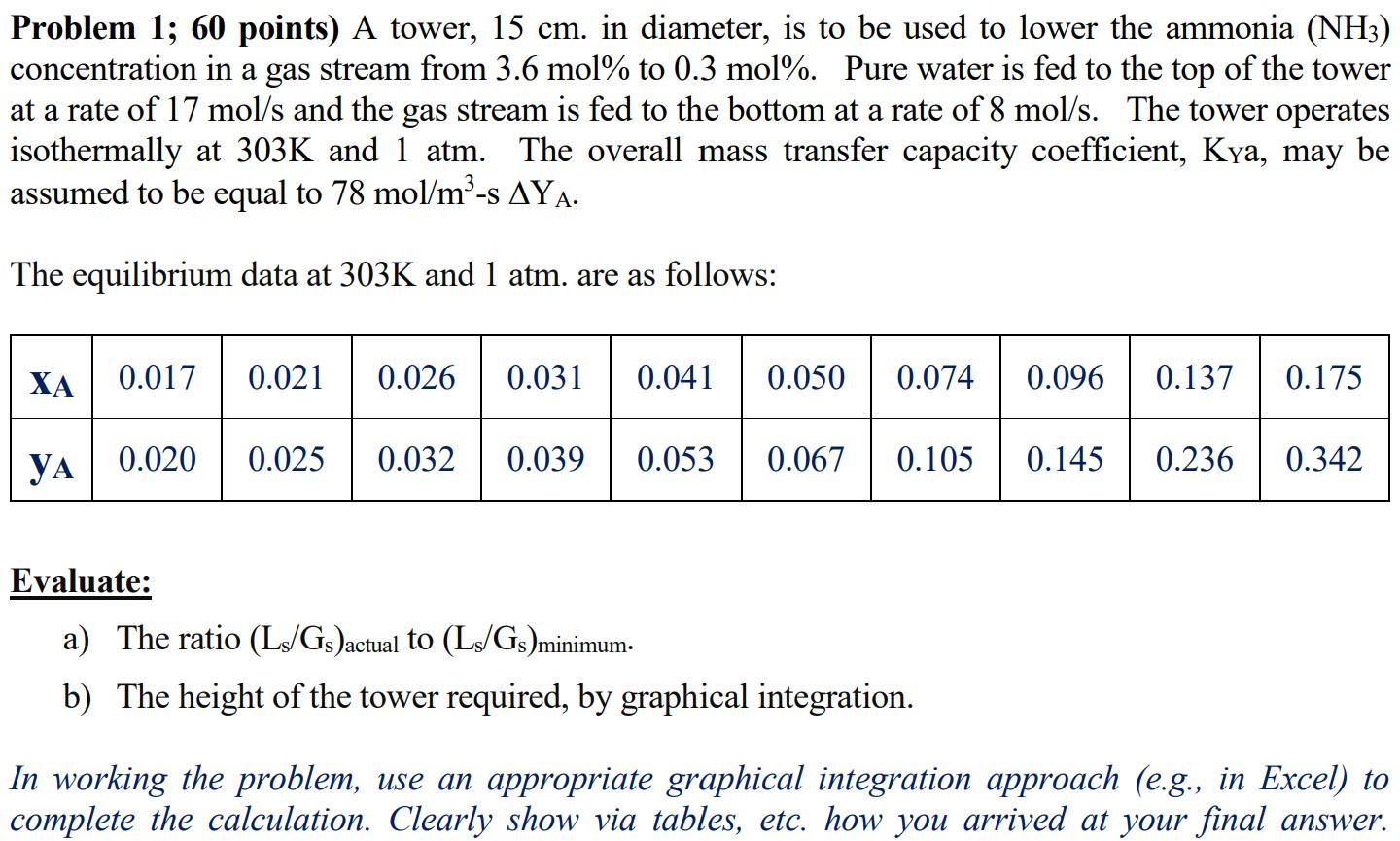
Problem 1; 60 points) A tower, 15 cm. in diameter, is to be used to lower the ammonia (NH3) concentration in a gas stream from 3.6 mol% to 0.3 mol%. Pure water is fed to the top of the tower at a rate of 17 mol/s and the gas stream is fed to the bottom at a rate of 8 mol/s. The tower operates isothermally at 303K and 1 atm. The overall mass transfer capacity coefficient, Kya, may be assumed to be equal to 78 mol/m-S AYA. The equilibrium data at 303K and 1 atm. are as follows: XA 0.017 0.021 0.026 0.031 0.041 0.050 0.074 0.096 0.137 0.175 0.020 0.025 0.032 0.039 0.053 0.067 0.105 0.145 0.236 0.342 Evaluate: a) The ratio (L3/Gs )actual to (L3/Gs)minimum. b) The height of the tower required, by graphical integration. In working the problem, use an appropriate graphical integration approach (e.g., in Excel) to complete the calculation. Clearly show via tables, etc. how you arrived at your final
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


