Question: Problem 1 A 200-dm constant-volume batch reactor is pressurized to 20 atm with a mixture of 75% A and 25% inert. The gas-phase reaction is
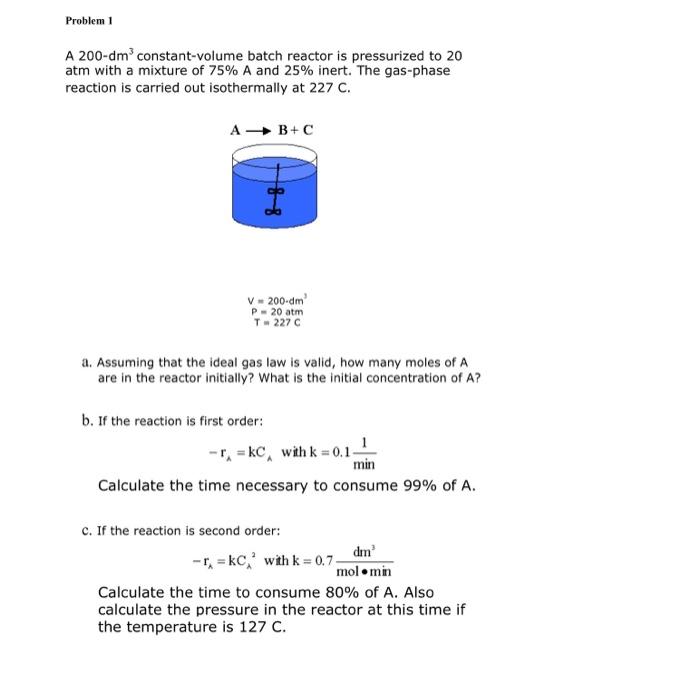
Problem 1 A 200-dm constant-volume batch reactor is pressurized to 20 atm with a mixture of 75% A and 25% inert. The gas-phase reaction is carried out isothermally at 227 C. AB+C V-200-cm P-20 atm T-227 C a. Assuming that the ideal gas law is valid, how many moles of A are in the reactor initially? What is the initial concentration of A? b. If the reaction is first order: -=kC, with k = 0.1- min Calculate the time necessary to consume 99% of A. C. If the reaction is second order: -C = kc, with k = 0.7 dm molomin Calculate the time to consume 80% of A. Also calculate the pressure in the reactor at this time if the temperature is 127 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


