Question: x is 4 . A 240 dm^3 constant volume batch. Time necessary to consume 94% of A. 3) A 2X0dm3 constant-volume batch reactor is pressurized
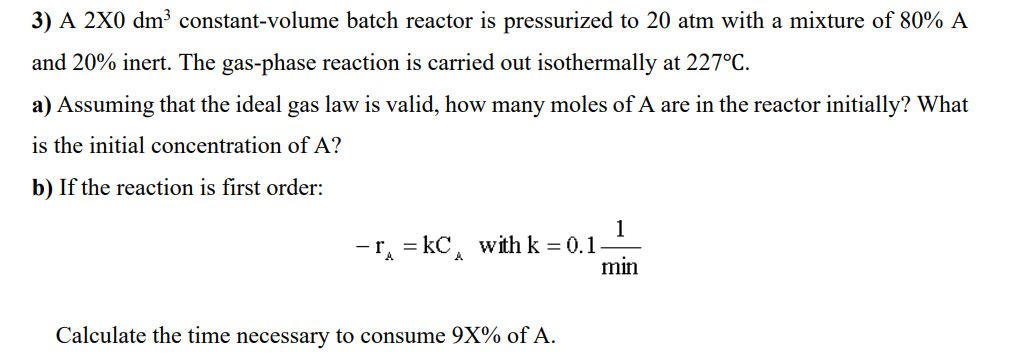 x is 4 . A 240 dm^3 constant volume batch. Time necessary to consume 94% of A.
x is 4 . A 240 dm^3 constant volume batch. Time necessary to consume 94% of A.
3) A 2X0dm3 constant-volume batch reactor is pressurized to 20atm with a mixture of 80%A and 20% inert. The gas-phase reaction is carried out isothermally at 227C. a) Assuming that the ideal gas law is valid, how many moles of A are in the reactor initially? What is the initial concentration of A ? b) If the reaction is first order: rA=kCAwithk=0.1min1 Calculate the time necessary to consume 9X% of A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


