Question: Problem #1: A gaseous mixture which is 70.0% methane, 20.0% propane, 5.0% carbon dioxide, and 5.0% oxygen by volume is burned with 100% dry excess
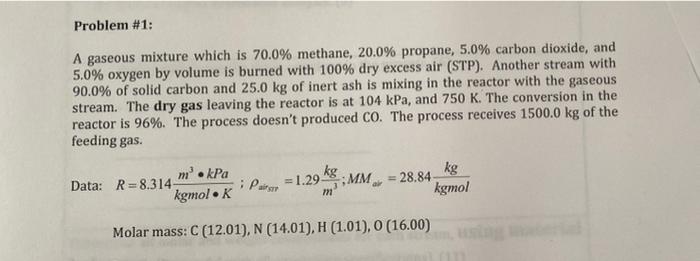
Problem #1: A gaseous mixture which is 70.0% methane, 20.0% propane, 5.0% carbon dioxide, and 5.0% oxygen by volume is burned with 100% dry excess air (STP). Another stream with 90.0% of solid carbon and 25.0 kg of inert ash is mixing in the reactor with the gaseous stream. The dry gas leaving the reactor is at 104 kPa, and 750 K. The conversion in the reactor is 96%. The process doesn't produced CO. The process receives 1500.0 kg of the feeding gas. m. kPa Data: R=8.314 kgmolK Pasto = 1.29 kg MM = 28.84 kg kgmol m Molar mass: C (12.01), N (14.01), H (1.01), O (16.00)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


