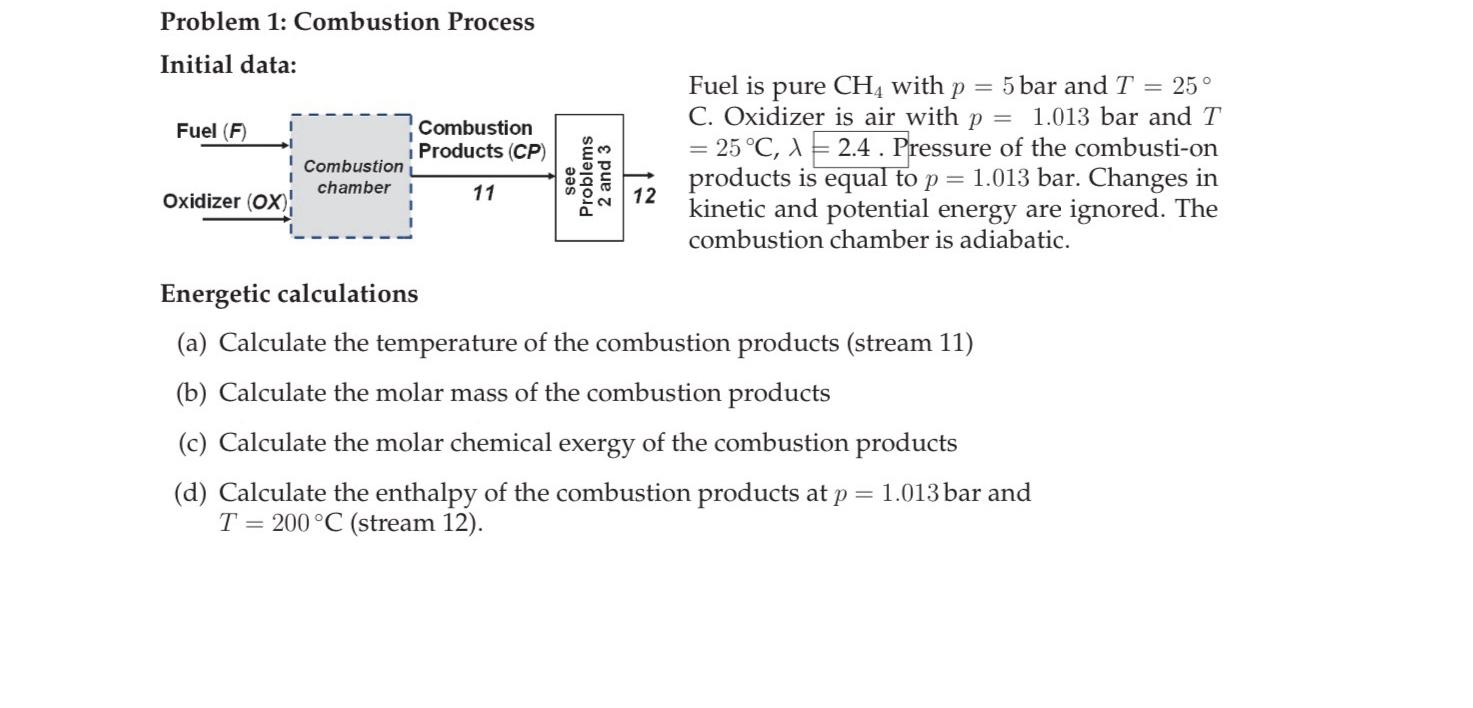
Question: Problem 1 : Combustion Process Initial data: Fuel is pure C H 4 with p = 5 bar and T = 2 5 C .
Problem : Combustion Process
Initial data:
Fuel is pure with bar and
C Oxidizer is air with bar and
Pressure of the combustion products is equal to bar. Changes in kinetic and potential energy are ignored. The combustion chamber is adiabatic.
Energetic calculations
a Calculate the temperature of the combustion products stream
b Calculate the molar mass of the combustion products
c Calculate the molar chemical exergy of the combustion products
d Calculate the enthalpy of the combustion products at bar and stream
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


