Question: Problem 1 (Constant volume Batch Reactor): The following reaction occurs in a constant-volume batch reactor: A+B==> R, with apparent rate law -1A = (1.8 M-1min-1)
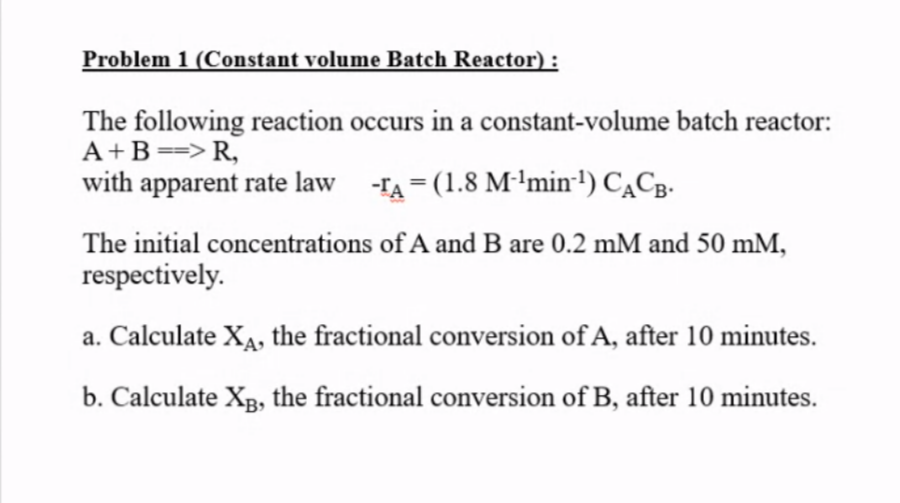
Problem 1 (Constant volume Batch Reactor): The following reaction occurs in a constant-volume batch reactor: A+B==> R, with apparent rate law -1A = (1.8 M-1min-1) CACB. . The initial concentrations of A and B are 0.2 mM and 50 mM, respectively. a. Calculate X, the fractional conversion of A, after 10 minutes. b. Calculate X3, the fractional conversion of B, after 10 minutes
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


