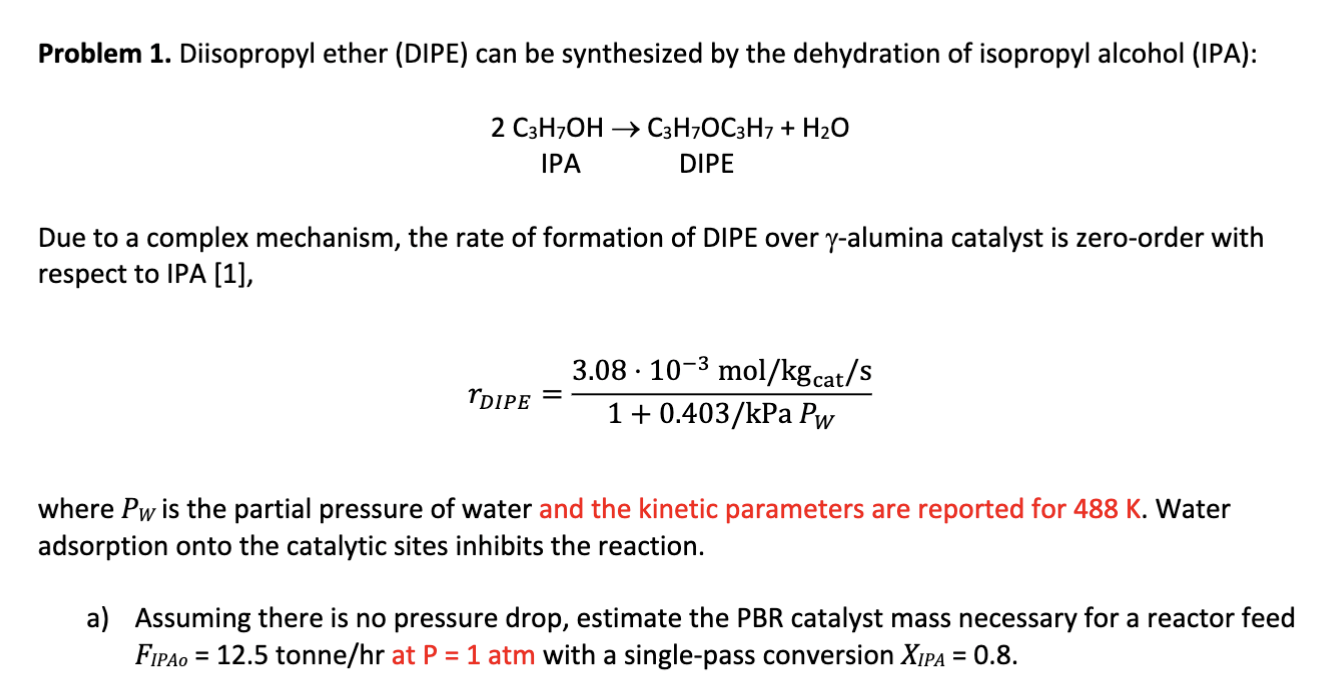
Question: Problem 1 . Diisopropyl ether ( DIPE ) can be synthesized by the dehydration of isopropyl alcohol ( IPA ) : 2 C 3 H
Problem Diisopropyl ether DIPE can be synthesized by the dehydration of isopropyl alcohol IPA:
Due to a complex mechanism, the rate of formation of DIPE over alumina catalyst is zeroorder with
respect to IPA
where is the partial pressure of water and the kinetic parameters are reported for Water
adsorption onto the catalytic sites inhibits the reaction.
a Assuming there is no pressure drop, estimate the PBR catalyst mass necessary for a reactor feed
tonne at atm with a singlepass conversion
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


