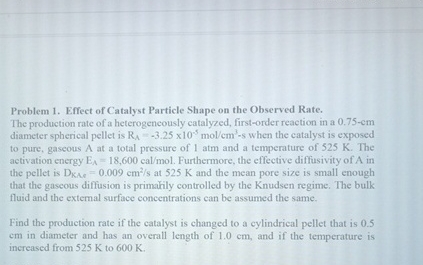
Question: Problem 1 . Effect of Catalyst Particle Shape on the Observed Rate. The production rate of a heterogencously catalyzed, first - order reaction in a
Problem Effect of Catalyst Particle Shape on the Observed Rate.
The production rate of a heterogencously catalyzed, firstorder reaction in a diameter spherical pellet is when the catalyst is exposed to pure, gaseous at a total pressure of atm and a temperature of The activation energy Furthermore, the effective diffusivity of in the pellet is at and the mean pore size is small enough that the gaseous diffusion is primatily controlled by the Knudsen regime. The bulk fluid and the extemal surface concentrations can be assumed the same.
Find the production rate if the catalyst is changed to a cylindrical pellet that is in diameter and has an overall length of and if the temperature is increased from to
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


