Question: Problem 1: Spinning electrodes are often used in electrochemistry experiments to reduce mass transfer limitation when measuring electrochemical reaction rate on various electrodes (Figure 1).
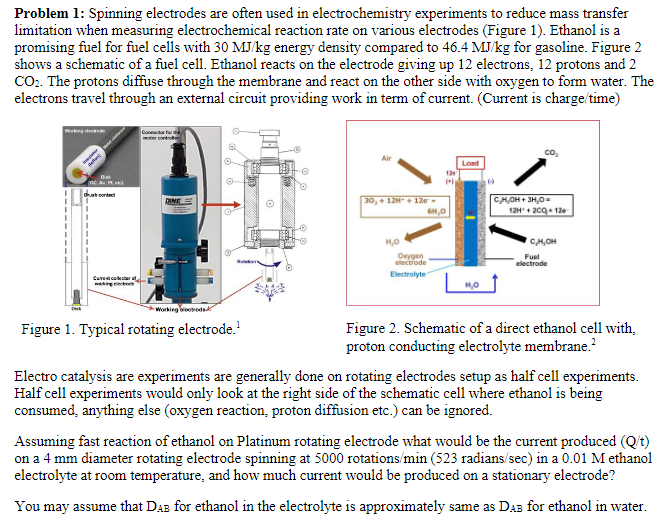
Problem 1: Spinning electrodes are often used in electrochemistry experiments to reduce mass transfer limitation when measuring electrochemical reaction rate on various electrodes (Figure 1). Ethanol is a promising fuel for fuel cells with 30MJ/kg energy density compared to 46.4MJ/kg for gasoline. Figure 2 shows a schematic of a fuel cell. Ethanol reacts on the electrode giving up 12 electrons, 12 protons and 2 CO2. The protons diffuse through the membrane and react on the other side with oxygen to form water. The electrons travel through an external circuit providing work in term of current. (Current is charge/time) Figure 1. Typical rotating electrode. 1 Figure 2. Schematic of a direct ethanol cell with, proton conducting electrolyte membrane. 2 Electro catalysis are experiments are generally done on rotating electrodes setup as half cell experiments. Half cell experiments would only look at the right side of the schematic cell where ethanol is being consumed, anything else (oxygen reaction, proton diffusion etc.) can be ignored. Assuming fast reaction of ethanol on Platinum rotating electrode what would be the current produced (Q/t) on a 4mm diameter rotating electrode spinning at 5000 rotations /min(523radians/sec) in a 0.01M ethanol electrolyte at room temperature, and how much current would be produced on a stationary electrode? You may assume that DAB for ethanol in the electrolyte is approximately same as DAB for ethanol in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


