Question: Problem 1 : The process shown below is the dehydrogenation of propane ( C 3 H 8 ) to propylene ( C 3 H 6
Problem :
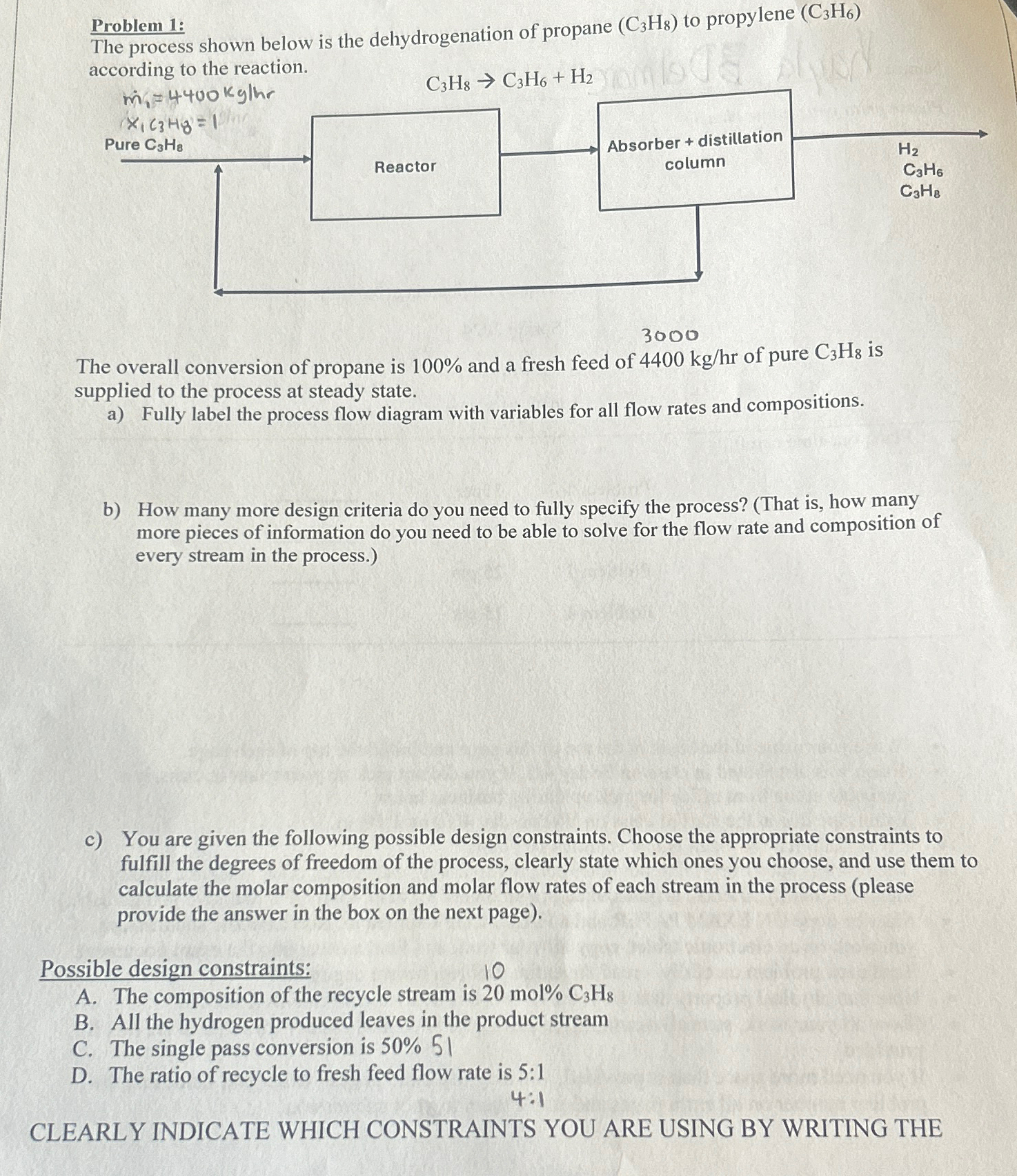
The process shown below is the dehydrogenation of propane to propylene according to the reaction.
The overall conversion of propane is and a fresh feed of of pure is supplied to the process at steady state.
a Fully label the process flow diagram with variables for all flow rates and compositions.
b How many more design criteria do you need to fully specify the process? That is how many more pieces of information do you need to be able to solve for the flow rate and composition of every stream in the process.
c You are given the following possible design constraints. Choose the appropriate constraints to fulfill the degrees of freedom of the process, clearly state which ones you choose, and use them to calculate the molar composition and molar flow rates of each stream in the process please provide the answer in the box on the next page
Possible design constraints:
A The composition of the recycle stream is mol
B All the hydrogen produced leaves in the product stream
C The single pass conversion is
D The ratio of recycle to fresh feed flow rate is :
:
CLEARLY INDICATE WHICH CONSTRAINTS YOU ARE USING BY WRITING THEM
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


