Question: Problem 1: (Thermodynamic effects) (25 points) Consider the Cu-Zn phase diagram given in the Figure below. 1. What type of invariant reactions occur in this
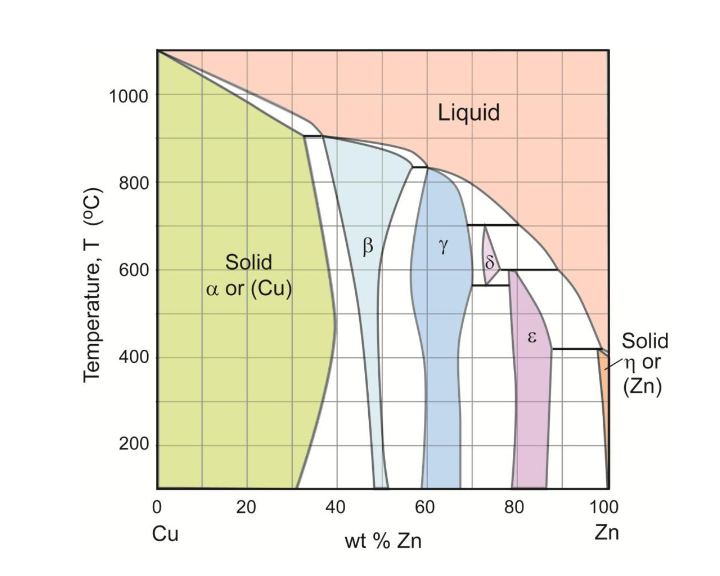
Problem 1: (Thermodynamic effects) (25 points) Consider the Cu-Zn phase diagram given in the Figure below. 1. What type of invariant reactions occur in this system? Show an example of each type of reaction. (6 points) 2. Draw a schematic plot of the free energy curves as a function of composition at the temperature 700 C. (9 points) 3. A liquid solution at 40 wt % Zn has been quenched to 700 C. Draw on the free energy curves the thermodynamic driving force for the transformation of the liquid to the solid phases. (5 points) 4. Is there a case of metastable equilibrium on the free energy curves you have drawn? If so, try to show it on the phase diagram at 700 C. If not, state why not. (5 points) 1000 Liquid 800 B y 600 Temperature, T (C) Solid a or (Cu) E Solid 400 in or (Zn) 200 20 80 0 Cu 40 60 wt% Zn 100 Zn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


