Question: Problem 1: Two substances A&B undergo a bimolecular reaction A+B Product. The following table gives the concentration of A at various times: The initial concentration
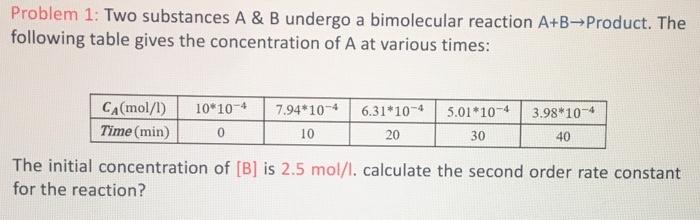
Problem 1: Two substances A&B undergo a bimolecular reaction A+B Product. The following table gives the concentration of A at various times: The initial concentration of [B] is 2.5mol/l. calculate the second order rate constant for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


