Question: Problem 1 (Warm up - 20 points): High temperature methanol vapor at 600C is needed for a chemical reaction. a) How much heat, in kJ/hr
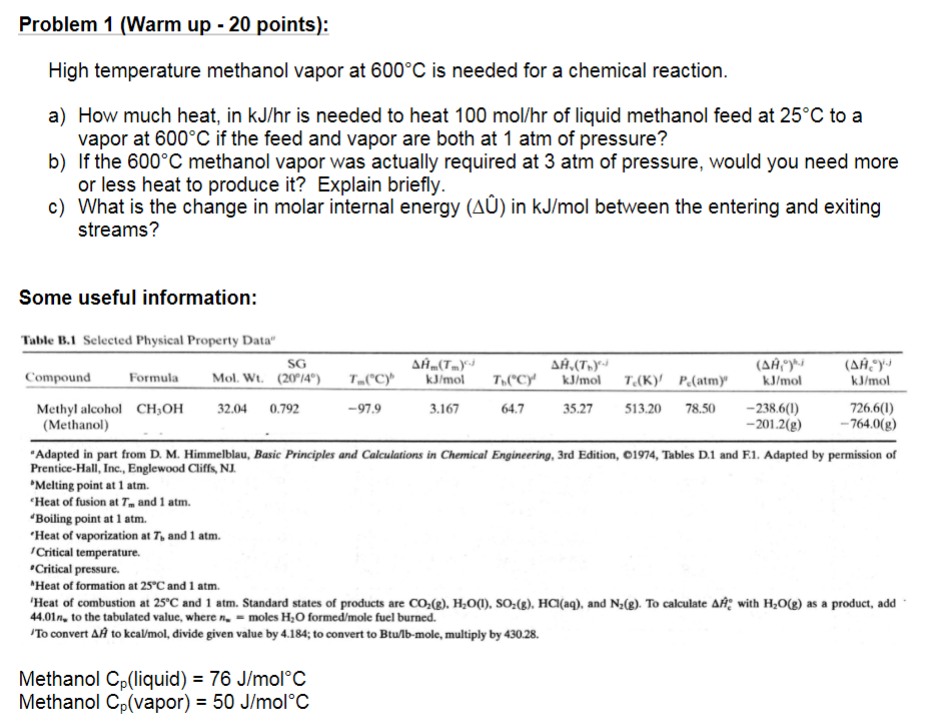
Problem 1 (Warm up - 20 points): High temperature methanol vapor at 600C is needed for a chemical reaction. a) How much heat, in kJ/hr is needed to heat 100 mol/hr of liquid methanol feed at 25C to a vapor at 600C if the feed and vapor are both at 1 atm of pressure? b) If the 600C methanol vapor was actually required at 3 atm of pressure, would you need more or less heat to produce it? Explain briefly. c) What is the change in molar internal energy (A) in kJ/mol between the entering and exiting streams? Some useful information: Table B.1 Selected Physical Property Data SG AH (1) ,(.y- (, y (,y Compound Formula Mol. Wt. (20/4) T.(C) kJ/mol T("C) kJ/mol T(K) P.(atm)' kJ/mol kJ/mol Methyl alcohol CH,OH 32.04 0.792 -979 3.167 64.7 35.27 513.20 78.50 -238.6(1) 726.6(1) (Methanol) -201.2(g) - 764.0(g) Adapted in part from D. M. Himmelblau, Basic Principles and Calculations in Chemical Engineering, 3rd Edition, C1974, Tables D.1 and F1. Adapted by permission of Prentice-Hall, Inc., Englewood Cliffs, NJ. Melting point at 1 atm. "Heat of fusion at T. and 1 atm. "Boiling point at 1 atm. 'Heat of vaporization at T, and 1 atm. Critical temperature Critical pressure "Heat of formation at 25C and 1 atm. 'Heat of combustion at 25C and 1 atm. Standard states of products are CO2(2). H2O(1), SO2(8). HCl(aq), and N2(2). To calculate All with H2O(2) as a product, add 44.01n, to the tabulated value, where - moles H2O formed/mole fuel burned. 'To convert AA to kcal/mol, divide given value by 4.184; to convert to Btulb-mole, multiply by 430.28. Methanol Cpliquid) = 76 J/molC Methanol Ci(vapor) = 50 J/molC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


