Question: Problem 1: What pH is required to reduce a high concentration of a dissolved Mg?* to 25 mg/L? The solubility product for the following reaction
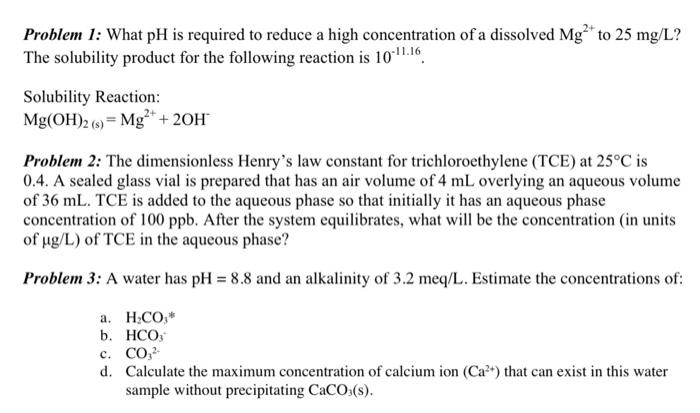
Problem 1: What pH is required to reduce a high concentration of a dissolved Mg?* to 25 mg/L? The solubility product for the following reaction is 10-11.16 Solubility Reaction: Mg(OH)2 (6) = Mg2+ + 20H Problem 2: The dimensionless Henry's law constant for trichloroethylene (TCE) at 25C is 0.4. A sealed glass vial is prepared that has an air volume of 4 mL overlying an aqueous volume of 36 mL. TCE is added to the aqueous phase so that initially it has an aqueous phase concentration of 100 ppb. After the system equilibrates, what will be the concentration (in units of ug/L) of TCE in the aqueous phase? Problem 3: A water has pH = 8.8 and an alkalinity of 3.2 meq/L. Estimate the concentrations of: a. H.CO,* b. HCO c. CO, d. Calculate the maximum concentration of calcium ion (Cat) that can exist in this water sample without precipitating CaCO3(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


