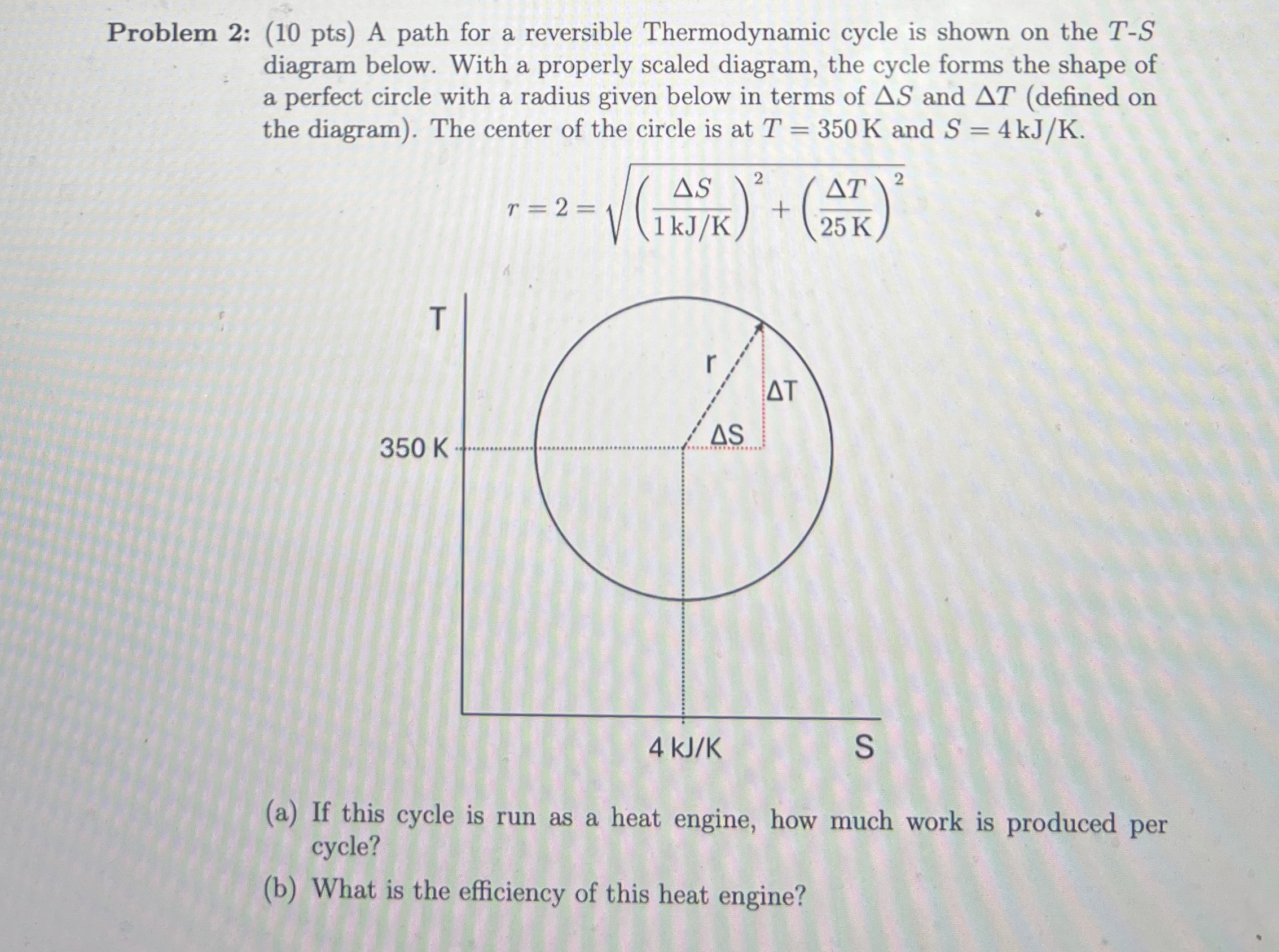
Question: Problem 2 : ( 1 0 pts ) A path for a reversible Thermodynamic cycle is shown on the T - S diagram below. With
Problem : pts A path for a reversible Thermodynamic cycle is shown on the diagram below. With a properly scaled diagram, the cycle forms the shape of a perfect circle with a radius given below in terms of and defined on the diagram The center of the circle is at and
a If this cycle is run as a heat engine, how much work is produced per cycle?
b What is the efficiency of this heat engine?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


