Question: Problem 2 (30 Pts) The heat capacity c is the number of heat units needed to raise the temperature of matter by one degree. The
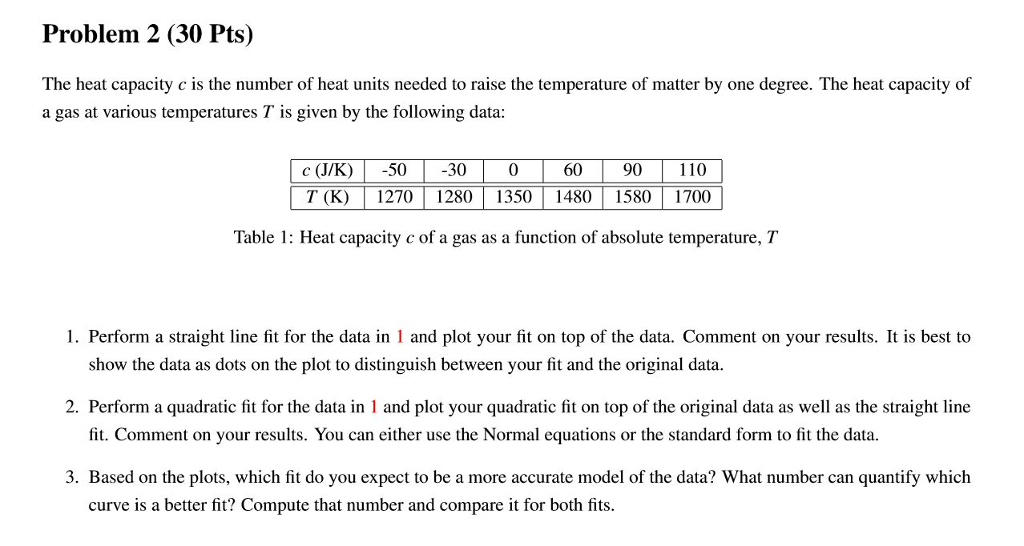
Problem 2 (30 Pts) The heat capacity c is the number of heat units needed to raise the temperature of matter by one degree. The heat capacity of a gas at various temperatures T is given by the following data: c (J/K)-50300 6090 110 T (K)1270 12801350 148015801700 Table 1: Heat capacity c of a gas as a function of absolute temperature, T 1. Perform a straight line fit for the data in 1 and plot your fit on top of the data. Comment on your results. It is best to show the data as dots on the plot to distinguish between your fit and the original data. 2. Perform a quadratic fit for the data in 1 and plot your quadratic fit on top of the original data as well as the straight line fit. Comment on your results. You can either use the Normal equations or the standard form to fit the data. model of the data? What num curve is a better fit? Compute that number and compare it for both fits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


